Pluripotent Stem Cells: Current Understanding and Future Directions
- PMID: 26798367
- PMCID: PMC4699068
- DOI: 10.1155/2016/9451492
Pluripotent Stem Cells: Current Understanding and Future Directions
Abstract
Pluripotent stem cells have the ability to undergo self-renewal and to give rise to all cells of the tissues of the body. However, this definition has been recently complicated by the existence of distinct cellular states that display these features. Here, we provide a detailed overview of the family of pluripotent cell lines derived from early mouse and human embryos and compare them with induced pluripotent stem cells. Shared and distinct features of these cells are reported as additional hallmark of pluripotency, offering a comprehensive scenario of pluripotent stem cells.
Figures

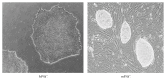
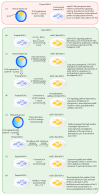


References
-
- Till J. E., McCulloch E. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiation Research. 1961;14:213–222. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

