Comparison of Immunomodulation Properties of Porcine Mesenchymal Stromal/Stem Cells Derived from the Bone Marrow, Adipose Tissue, and Dermal Skin Tissue
- PMID: 26798368
- PMCID: PMC4699062
- DOI: 10.1155/2016/9581350
Comparison of Immunomodulation Properties of Porcine Mesenchymal Stromal/Stem Cells Derived from the Bone Marrow, Adipose Tissue, and Dermal Skin Tissue
Abstract
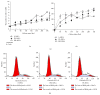
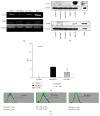
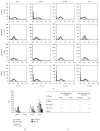
Mesenchymal stromal/stem cells (MSCs) demonstrate immunomodulation capacity that has been implicated in the reduction of graft-versus-host disease. Accordingly, we herein investigated the capacity of MSCs derived from several tissue sources to modulate both proinflammatory (interferon [IFN] γ and tumor necrosis factor [TNF] α) and immunosuppressive cytokines (transforming growth factor [TGF] β and interleukin [IL] 10) employing xenogeneic human MSC-mixed lymphocyte reaction (MLR) test. Bone marrow-derived MSCs showed higher self-renewal capacity with relatively slow proliferation rate in contrast to adipose-derived MSCs which displayed higher proliferation rate. Except for the lipoprotein gene, there were no marked changes in osteogenesis- and adipogenesis-related genes following in vitro differentiation; however, the histological marker analysis revealed that adipose MSCs could be differentiated into both adipose and bone tissue. TGFβ and IL10 were detected in adipose MSCs and bone marrow MSCs, respectively. However, skin-derived MSCs expressed both IFNγ and IL10, which may render them sensitive to immunomodulation. The xenogeneic human MLR test revealed that MSCs had a partial immunomodulation capacity, as proliferation of activated and resting peripheral blood mononuclear cells was not affected, but this did not differ among MSC sources. MSCs were not tumorigenic when introduced into immunodeficient mice. We concluded that the characteristics of MSCs are tissue source-dependent and their in vivo application requires more in-depth investigation regarding their precise immunomodulation capacities.
Figures




Similar articles
-
Comparative analysis of human mesenchymal stem cells from fetal-bone marrow, adipose tissue, and Warton's jelly as sources of cell immunomodulatory therapy.Hum Vaccin Immunother. 2016;12(1):85-96. doi: 10.1080/21645515.2015.1030549. Hum Vaccin Immunother. 2016. PMID: 26186552 Free PMC article.
-
IFN-γ Licensing Does Not Enhance the Reduced Immunomodulatory Potential and Migratory Ability of Differentiation-Induced Porcine Bone Marrow-Derived Mesenchymal Stem Cells in an In Vitro Xenogeneic Application.Biomed Res Int. 2021 Sep 4;2021:4604856. doi: 10.1155/2021/4604856. eCollection 2021. Biomed Res Int. 2021. PMID: 34527737 Free PMC article.
-
The effect of pro-inflammatory cytokines on immunophenotype, differentiation capacity and immunomodulatory functions of human mesenchymal stem cells.Cytokine. 2016 Sep;85:51-60. doi: 10.1016/j.cyto.2016.06.003. Epub 2016 Jun 9. Cytokine. 2016. PMID: 27288632
-
Immunosuppressive properties of mesenchymal stem cells: advances and applications.Curr Mol Med. 2012 Jun;12(5):574-91. doi: 10.2174/156652412800619950. Curr Mol Med. 2012. PMID: 22515979 Review.
-
Inflammatory Mediators in Glioma Microenvironment Play a Dual Role in Gliomagenesis and Mesenchymal Stem Cell Homing: Implication for Cellular Therapy.Mayo Clin Proc Innov Qual Outcomes. 2020 Aug 5;4(4):443-459. doi: 10.1016/j.mayocpiqo.2020.04.006. eCollection 2020 Aug. Mayo Clin Proc Innov Qual Outcomes. 2020. PMID: 32793872 Free PMC article. Review.
Cited by
-
Research Status of Mesenchymal Stem Cells in Liver Transplantation.Cell Transplant. 2019 Dec;28(12):1490-1506. doi: 10.1177/0963689719874786. Epub 2019 Sep 12. Cell Transplant. 2019. PMID: 31512503 Free PMC article. Review.
-
Bone marrow stromal cells improved functional recovery in spinal cord injury rats partly via the Toll-like receptor-4/nuclear factor-κB signaling pathway.Exp Ther Med. 2019 Jan;17(1):444-448. doi: 10.3892/etm.2018.6907. Epub 2018 Oct 31. Exp Ther Med. 2019. PMID: 30651819 Free PMC article.
-
Bone marrow donor selection and characterization of MSCs is critical for pre-clinical and clinical cell dose production.J Transl Med. 2019 Apr 17;17(1):128. doi: 10.1186/s12967-019-1877-4. J Transl Med. 2019. PMID: 30995929 Free PMC article.
-
Properties of porcine adipose-derived stem cells and their applications in preclinical models.Adipocyte. 2017 Jul 3;6(3):217-223. doi: 10.1080/21623945.2017.1312040. Epub 2017 Mar 30. Adipocyte. 2017. PMID: 28410000 Free PMC article. Review.
-
Osteogenic differentiation potential of porcine bone marrow mesenchymal stem cell subpopulations selected in different basal media.Biol Open. 2020 Oct 19;9(10):bio053280. doi: 10.1242/bio.053280. Biol Open. 2020. PMID: 32973080 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

