Role of the Lipid Environment in the Dimerization of Transmembrane Domains of Glycophorin A
- PMID: 26798499
- PMCID: PMC4717257
Role of the Lipid Environment in the Dimerization of Transmembrane Domains of Glycophorin A
Abstract
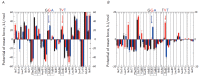
An efficient computational approach is developed to quantify the free energy of a spontaneous association of the α-helices of proteins in the membrane environment. The approach is based on the numerical decomposition of the free energy profiles of the transmembrane (TM) helices into components corresponding to protein-protein, protein-lipid, and protein-water interactions. The method was tested for the TM segments of human glycophorin A (GpA) and two mutant forms, Gly83Ala and Thr87Val. It was shown that lipids make a significant negative contribution to the free energy of dimerization, while amino acid residues forming the interface of the helix-helix contact may be unfavorable in terms of free energy. The detailed balance between different energy contributions is highly dependent on the amino acid sequence of the TM protein segment. The results show the dominant role of the environment in the interaction of membrane proteins that is changing our notion of the driving force behind the spontaneous association of TM α-helices. Adequate estimation of the contribution of the water-lipid environment thus becomes an extremely urgent task for a rational design of new molecules targeting bitopic membrane proteins, including receptor tyrosine kinases.
Keywords: free energy of intermolecular interactions; glycophorin A; molecular dynamics; protein-protein interactions; role of the lipid membrane; transmembrane domain.
Figures

References
LinkOut - more resources
Full Text Sources
