Bone tissue engineering scaffolding: computer-aided scaffolding techniques
- PMID: 26798575
- PMCID: PMC4709372
- DOI: 10.1007/s40204-014-0026-7
Bone tissue engineering scaffolding: computer-aided scaffolding techniques
Abstract
Tissue engineering is essentially a technique for imitating nature. Natural tissues consist of three components: cells, signalling systems (e.g. growth factors) and extracellular matrix (ECM). The ECM forms a scaffold for its cells. Hence, the engineered tissue construct is an artificial scaffold populated with living cells and signalling molecules. A huge effort has been invested in bone tissue engineering, in which a highly porous scaffold plays a critical role in guiding bone and vascular tissue growth and regeneration in three dimensions. In the last two decades, numerous scaffolding techniques have been developed to fabricate highly interconnective, porous scaffolds for bone tissue engineering applications. This review provides an update on the progress of foaming technology of biomaterials, with a special attention being focused on computer-aided manufacturing (Andrade et al. 2002) techniques. This article starts with a brief introduction of tissue engineering (Bone tissue engineering and scaffolds) and scaffolding materials (Biomaterials used in bone tissue engineering). After a brief reviews on conventional scaffolding techniques (Conventional scaffolding techniques), a number of CAM techniques are reviewed in great detail. For each technique, the structure and mechanical integrity of fabricated scaffolds are discussed in detail. Finally, the advantaged and disadvantage of these techniques are compared (Comparison of scaffolding techniques) and summarised (Summary).
Keywords: Bioceramics; Bone tissue engineering; Computer-aided scaffolding techniques; Scaffold; Solid free-form fabrication.
Figures













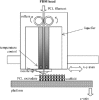

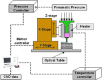

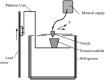

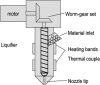






Similar articles
-
Current state of fabrication technologies and materials for bone tissue engineering.Acta Biomater. 2018 Oct 15;80:1-30. doi: 10.1016/j.actbio.2018.09.031. Epub 2018 Sep 22. Acta Biomater. 2018. PMID: 30248515 Review.
-
Three-dimensional (3D) printed scaffold and material selection for bone repair.Acta Biomater. 2019 Jan 15;84:16-33. doi: 10.1016/j.actbio.2018.11.039. Epub 2018 Nov 24. Acta Biomater. 2019. PMID: 30481607 Review.
-
Bone tissue engineering: Scaffold preparation using chitosan and other biomaterials with different design and fabrication techniques.Int J Biol Macromol. 2018 Nov;119:1228-1239. doi: 10.1016/j.ijbiomac.2018.08.056. Epub 2018 Aug 11. Int J Biol Macromol. 2018. PMID: 30107161 Review.
-
Bone Tissue Engineering Scaffolds: Materials and Methods.3D Print Addit Manuf. 2024 Feb 1;11(1):347-362. doi: 10.1089/3dp.2022.0216. Epub 2024 Feb 15. 3D Print Addit Manuf. 2024. PMID: 38389691 Free PMC article. Review.
-
[Development of computer aided forming techniques in manufacturing scaffolds for bone tissue engineering].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011 Dec;25(12):1508-12. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011. PMID: 22242356 Review. Chinese.
Cited by
-
Biomimetic, mussel-inspired surface modification of 3D-printed biodegradable polylactic acid scaffolds with nano-hydroxyapatite for bone tissue engineering.Front Bioeng Biotechnol. 2022 Sep 8;10:989729. doi: 10.3389/fbioe.2022.989729. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36159699 Free PMC article.
-
Development of Composite Scaffolds Based on Cerium Doped-Hydroxyapatite and Natural Gums-Biological and Mechanical Properties.Materials (Basel). 2019 Jul 26;12(15):2389. doi: 10.3390/ma12152389. Materials (Basel). 2019. PMID: 31357470 Free PMC article.
-
Biphasic Bioceramic Obtained from Byproducts of Sugar Beet Processing for Use in Bioactive Coatings and Bone Fillings.J Funct Biomater. 2023 Oct 9;14(10):499. doi: 10.3390/jfb14100499. J Funct Biomater. 2023. PMID: 37888165 Free PMC article.
-
Fused Deposition Modeling of ABS-Barium Titanate Composites: A Simple Route towards Tailored Dielectric Devices.Polymers (Basel). 2018 Jun 14;10(6):666. doi: 10.3390/polym10060666. Polymers (Basel). 2018. PMID: 30966700 Free PMC article.
-
3D and 4D Printing of Polymers for Tissue Engineering Applications.Front Bioeng Biotechnol. 2019 Jul 9;7:164. doi: 10.3389/fbioe.2019.00164. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31338366 Free PMC article. Review.
References
-
- Chen A, Tsang V, Albrecht D, Bhatia S (2007) 3-D fabrication technology for tissue engineering. In: Ferrari M, Desai T, Bhatia S (eds) BioMEMS and biomedical nanotechnology. Springer, Berlin, pp 23–38
-
- Oliveira AL, Costa SA, Sousa RA, Reis RL (2009) Nucleation and growth of biomimetic apatite layers on 3D plotted biodegradable polymeric scaffolds: effect of static and dynamic coating conditions. Acta Biomaterialia 5:1626–1638 - PubMed
-
- Lode A, Meissner K, Luo Y, Sonntag F, Glorius S, Nies B, Vater C, Despang F, Hanke T, Gelinsky M (2012) Fabrication of porous scaffolds by three-dimensional plotting of a pasty calcium phosphate bone cement under mild conditions. J Tissue Eng Regenerat Med - PubMed
-
- Martins A, Chung S, Pedro AJ, Sousa RA, Marques AP, Reis RL, Neves NM. Hierarchical starch-based fibrous scaffold for bone tissue engineering applications. J Tissue Eng Regenerat Med. 2009;3:37–42. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

