Lessons from Animal Models of Arterial Aneurysm
- PMID: 26798701
- PMCID: PMC4682768
- DOI: 10.12945/j.aorta.2013.13-052
Lessons from Animal Models of Arterial Aneurysm
Abstract
We review the results from the most common animal models of arterial aneurysm, including recent findings from our novel, laparoscopy-based pig model of abdominal aortic aneurysm, that contribute important insights into early pathogenesis. We emphasize the relevance of these findings for evaluation of treatment protocols and novel device prototypes for mechanism-based prevention of progression and rupture.
Keywords: Abdominal aortic aneurysm; Animal models; Arterial aneurysm; Periarterial calcium chloride.
Figures


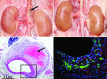
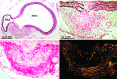
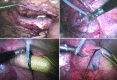
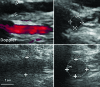


Similar articles
-
Animal models of abdominal aortic aneurysm and their role in furthering management of human disease.Cardiovasc Pathol. 2011 Mar-Apr;20(2):114-23. doi: 10.1016/j.carpath.2010.01.001. Epub 2010 Feb 4. Cardiovasc Pathol. 2011. PMID: 20133168 Review.
-
Murine aortic aneurysm produced by periarterial application of calcium chloride.J Surg Res. 2001 Aug;99(2):371-6. doi: 10.1006/jsre.2001.6207. J Surg Res. 2001. PMID: 11469913
-
Endovascular model of abdominal aortic aneurysm induction in swine.Vasc Med. 2014 Jun;19(3):167-174. doi: 10.1177/1358863X14534006. Epub 2014 May 30. Vasc Med. 2014. PMID: 24879711
-
Different long-term outcomes of abdominal aortic aneurysm and intracranial aneurysm models: hemodynamic change may also play an essential role in the initiation and progression of abdominal aortic aneurysm in rabbits.Cell Biochem Biophys. 2014 Nov;70(2):819-22. doi: 10.1007/s12013-014-9985-5. Cell Biochem Biophys. 2014. PMID: 24801772
-
Recent Advances in the Development of Experimental Animal Models Mimicking Human Aortic Aneurysms.Vasc Specialist Int. 2015 Mar;31(1):1-10. doi: 10.5758/vsi.2015.31.1.1. Epub 2015 Mar 31. Vasc Specialist Int. 2015. PMID: 26217637 Free PMC article. Review.
Cited by
-
Computational Fluid Dynamics of Vascular Disease in Animal Models.J Biomech Eng. 2018 Aug 1;140(8):0808011-08080114. doi: 10.1115/1.4039678. J Biomech Eng. 2018. PMID: 29570754 Free PMC article. Review.
-
Abdominal Aortic Aneurysm: Evolving Controversies and Uncertainties.Int J Angiol. 2018 Jun;27(2):58-80. doi: 10.1055/s-0038-1657771. Epub 2018 May 29. Int J Angiol. 2018. PMID: 29896039 Free PMC article. Review.
-
Thrombin-Fibrinogen In Vitro Flow Model of Thrombus Growth in Cerebral Aneurysms.TH Open. 2021 May 12;5(2):e155-e162. doi: 10.1055/s-0041-1728790. eCollection 2021 Apr. TH Open. 2021. PMID: 34007954 Free PMC article.
-
Translating mouse models of abdominal aortic aneurysm to the translational needs of vascular surgery.JVS Vasc Sci. 2021 Mar 3;2:219-234. doi: 10.1016/j.jvssci.2021.01.002. eCollection 2021. JVS Vasc Sci. 2021. PMID: 34778850 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

