Acute Infections, Cost per Infection and Turnaround Time in Three United States Hospital Laboratories Using Fourth-Generation Antigen-Antibody Human Immunodeficiency Virus Immunoassays
- PMID: 26798766
- PMCID: PMC4719082
- DOI: 10.1093/ofid/ofv188
Acute Infections, Cost per Infection and Turnaround Time in Three United States Hospital Laboratories Using Fourth-Generation Antigen-Antibody Human Immunodeficiency Virus Immunoassays
Abstract
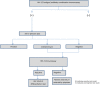
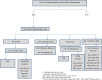
Background. To improve clinical and public health outcomes through early human immunodeficiency virus (HIV) detection, fourth-generation antigen/antibody immunoassay (4IA) and supplemental testing results must be returned rapidly. Methods. We examined HIV testing data at Harborview Medical Center (HMC), Massachusetts General Hospital (MGH), and the Medical University of South Carolina (MUSC), which used 4IA and supplemental antibody and nucleic acid tests (NATs). At MGH and MUSC, HIV-1 Western blot (WB) and HIV-2 testing were conducted at a reference laboratory. We compared time from specimen collection to laboratory result for established (positive WB) and acute infections (reactive 4IA, negative/indeterminate WB, detectable NAT), and we calculated testing cost per positive-test result. Results. From 3731 (MUSC) to 19 774 (MGH) tests were conducted; 0.01% (MGH) to 0.05% (HMC) were acute infections. Each laboratory had reactive 4IA, WB-negative, or indeterminate specimens without NAT (ie, potential acute infections). Time to result was 1.5 (HMC) to 5.2 days (MGH) for acute and 1.0 (HMC) to 5.2 days (MGH) for established infections. Costs were $1054 (MGH) to $1521 (MUSC). Conclusions. Conducting supplemental testing in-house lowered turnaround times, which may be further reduced with rapid HIV-1/HIV-2 differentiation tests. Hospitals may benefit from quantitative NATs not requiring physician orders, so all potential acute infections receive NAT.
Keywords: HIV; acute infection; cost; laboratory.
Figures
References
-
- Daar ES, Moudgil T, Meyer RD, Ho DD. Transient high levels of viremia in patients with primary human immunodeficiency virus type 1 infection. N Engl J Med 1991; 324:961–4. - PubMed
-
- Dowshen N, Pierce VM, Zanno A et al. . Acute HIV infection in a critically ill 15-year-old male. Pediatrics 2013; 131:e959–63. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources