Real-time investigation of dynamic protein crystallization in living cells
- PMID: 26798811
- PMCID: PMC4711630
- DOI: 10.1063/1.4921591
Real-time investigation of dynamic protein crystallization in living cells
Abstract
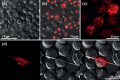
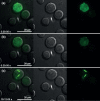
X-ray crystallography requires sufficiently large crystals to obtain structural insights at atomic resolution, routinely obtained in vitro by time-consuming screening. Recently, successful data collection was reported from protein microcrystals grown within living cells using highly brilliant free-electron laser and third-generation synchrotron radiation. Here, we analyzed in vivo crystal growth of firefly luciferase and Green Fluorescent Protein-tagged reovirus μNS by live-cell imaging, showing that dimensions of living cells did not limit crystal size. The crystallization process is highly dynamic and occurs in different cellular compartments. In vivo protein crystallization offers exciting new possibilities for proteins that do not form crystals in vitro.
Figures




Similar articles
-
In vivo protein crystallization in combination with highly brilliant radiation sources offers novel opportunities for the structural analysis of post-translationally modified eukaryotic proteins.Acta Crystallogr F Struct Biol Commun. 2015 Aug;71(Pt 8):929-37. doi: 10.1107/S2053230X15011450. Epub 2015 Jul 29. Acta Crystallogr F Struct Biol Commun. 2015. PMID: 26249677 Free PMC article. Review.
-
Enhanced X-ray diffraction of in vivo-grown μNS crystals by viscous jets at XFELs.Acta Crystallogr F Struct Biol Commun. 2020 Jun 1;76(Pt 6):278-289. doi: 10.1107/S2053230X20006172. Epub 2020 May 29. Acta Crystallogr F Struct Biol Commun. 2020. PMID: 32510469 Free PMC article.
-
A streamlined approach to structure elucidation using in cellulo crystallized recombinant proteins, InCellCryst.Nat Commun. 2024 Feb 24;15(1):1709. doi: 10.1038/s41467-024-45985-7. Nat Commun. 2024. PMID: 38402242 Free PMC article.
-
Preparation and Delivery of Protein Microcrystals in Lipidic Cubic Phase for Serial Femtosecond Crystallography.J Vis Exp. 2016 Sep 20;(115):54463. doi: 10.3791/54463. J Vis Exp. 2016. PMID: 27683972 Free PMC article.
-
Protein crystallization in living cells.Biol Chem. 2018 Jun 27;399(7):751-772. doi: 10.1515/hsz-2018-0158. Biol Chem. 2018. PMID: 29894295 Review.
Cited by
-
A pipeline for structure determination of in vivo-grown crystals using in cellulo diffraction.Acta Crystallogr D Struct Biol. 2016 Apr;72(Pt 4):576-85. doi: 10.1107/S2059798316002369. Epub 2016 Mar 30. Acta Crystallogr D Struct Biol. 2016. PMID: 27050136 Free PMC article.
-
Review of serial femtosecond crystallography including the COVID-19 pandemic impact and future outlook.Structure. 2023 Nov 2;31(11):1306-1319. doi: 10.1016/j.str.2023.10.005. Epub 2023 Oct 27. Structure. 2023. PMID: 37898125 Free PMC article. Review.
-
Correlation between Renal Artery Anatomy and Hypertension: A Retrospective Analysis of 3000 Patients.Evid Based Complement Alternat Med. 2021 Dec 29;2021:9957361. doi: 10.1155/2021/9957361. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 35003315 Free PMC article.
-
Production and Isolation of Magnetic Protein Crystals in HEK293T Cells.Bio Protoc. 2020 Jul 20;10(14):e3684. doi: 10.21769/BioProtoc.3684. eCollection 2020 Jul 20. Bio Protoc. 2020. PMID: 33659355 Free PMC article.
-
In vivo protein crystallization in combination with highly brilliant radiation sources offers novel opportunities for the structural analysis of post-translationally modified eukaryotic proteins.Acta Crystallogr F Struct Biol Commun. 2015 Aug;71(Pt 8):929-37. doi: 10.1107/S2053230X15011450. Epub 2015 Jul 29. Acta Crystallogr F Struct Biol Commun. 2015. PMID: 26249677 Free PMC article. Review.
References
-
- Henderson R., “ Cryoprotection of protein crystals against radiation damage in electron and X-ray diffraction,” Proc. R. Soc. London, Ser. B 241, 6–8 (1990).10.1098/rspb.1990.0057 - DOI
-
- Garman E. F. and Schneider T. R., “ Macromolecular Cryocrystallography,” J. Appl. Crystallogr. 30, 211–237 (1997).10.1107/S0021889897002677 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

