A liquid flatjet system for solution phase soft-x-ray spectroscopy
- PMID: 26798824
- PMCID: PMC4711648
- DOI: 10.1063/1.4928715
A liquid flatjet system for solution phase soft-x-ray spectroscopy
Abstract
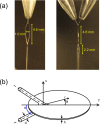
We present a liquid flatjet system for solution phase soft-x-ray spectroscopy. The flatjet set-up utilises the phenomenon of formation of stable liquid sheets upon collision of two identical laminar jets. Colliding the two single water jets, coming out of the nozzles with 50 μm orifices, under an impact angle of 48° leads to double sheet formation, of which the first sheet is 4.6 mm long and 1.0 mm wide. The liquid flatjet operates fully functional under vacuum conditions (<10(-3) mbar), allowing soft-x-ray spectroscopy of aqueous solutions in transmission mode. We analyse the liquid water flatjet thickness under atmospheric pressure using interferomeric or mid-infrared transmission measurements and under vacuum conditions by measuring the absorbance of the O K-edge of water in transmission, and comparing our results with previously published data obtained with standing cells with Si3N4 membrane windows. The thickness of the first liquid sheet is found to vary between 1.4-3 μm, depending on the transverse and longitudinal position in the liquid sheet. We observe that the derived thickness is of similar magnitude under 1 bar and under vacuum conditions. A catcher unit facilitates the recycling of the solutions, allowing measurements on small sample volumes (∼10 ml). We demonstrate the applicability of this approach by presenting measurements on the N K-edge of aqueous NH4 (+). Our results suggest the high potential of using liquid flatjets in steady-state and time-resolved studies in the soft-x-ray regime.
Figures






References
-
- Brown M. A., Arrigoni M., Heroguel F., Redondo A. B., Giordano L., van Bokhoven J. A., and Pacchioni G., J. Phys. Chem. C 118, 29007 (2014).10.1021/jp502262f - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

