The FgNot3 Subunit of the Ccr4-Not Complex Regulates Vegetative Growth, Sporulation, and Virulence in Fusarium graminearum
- PMID: 26799401
- PMCID: PMC4723064
- DOI: 10.1371/journal.pone.0147481
The FgNot3 Subunit of the Ccr4-Not Complex Regulates Vegetative Growth, Sporulation, and Virulence in Fusarium graminearum
Abstract
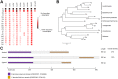
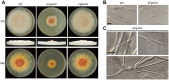
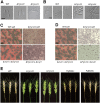
The Ccr4-Not complex is evolutionarily conserved and important for multiple cellular functions in eukaryotic cells. In this study, the biological roles of the FgNot3 subunit of this complex were investigated in the plant pathogenic fungus Fusarium graminearum. Deletion of FgNOT3 resulted in retarded vegetative growth, retarded spore germination, swollen hyphae, and hyper-branching. The ΔFgnot3 mutants also showed impaired sexual and asexual sporulation, decreased virulence, and reduced expression of genes related to conidiogenesis. Fgnot3 deletion mutants were sensitive to thermal stress, whereas NOT3 orthologs in other model eukaryotes are known to be required for cell wall integrity. We found that FgNot3 functions as a negative regulator of the production of secondary metabolites, including trichothecenes and zearalenone. Further functional characterization of other components of the Not module of the Ccr4-Not complex demonstrated that the module is conserved. Each subunit primarily functions within the context of a complex and might have distinct roles outside of the complex in F. graminearum. This is the first study to functionally characterize the Not module in filamentous fungi and provides novel insights into signal transduction pathways in fungal development.
Conflict of interest statement
Figures



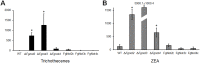
References
-
- Sutton J. Epidemiology of wheat head blight and maize ear rot caused by Fusarium graminearum. Can J Plant Pathol. 1982; 4: 195–209.
-
- Desjardins AE. Fusarium mycotoxins: chemistry, genetics, and biology. St Paul, MN: American Phytopathological Society Press; 2006.
-
- Leslie JF, Summerell BA, Bullock S. The Fusarium laboratory manual. vol 10 Ames, IA: Blackwee Pub; 2006.
-
- Guenther JC, Trail F. The development and differentiation of Gibberella zeae (anamorph: Fusarium graminearum) during colonization of wheat. Mycologia. 2005; 97: 229–237. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

