A 24-week study to evaluate the efficacy and safety of once-weekly dulaglutide added on to glimepiride in type 2 diabetes (AWARD-8)
- PMID: 26799540
- PMCID: PMC5067625
- DOI: 10.1111/dom.12634
A 24-week study to evaluate the efficacy and safety of once-weekly dulaglutide added on to glimepiride in type 2 diabetes (AWARD-8)
Abstract
Aims: To evaluate the safety and efficacy of once-weekly dulaglutide 1.5 mg, a long-acting glucagon-like peptide-1 receptor agonist, compared with placebo in patients with type 2 diabetes (T2D) on glimepiride monotherapy.
Methods: This phase III, randomized (4 : 1; dulaglutide:placebo), double-blind, placebo-controlled, 24-week study compared the safety and efficacy of once-weekly dulaglutide 1.5 mg with placebo in sulphonylurea-treated (≥half-maximal dose, stable ≥3 months) patients (N = 300) with T2D and inadequate glycaemic control [glycated haemoglobin (HbA1c) ≥7.5 and ≤9.5% (≥58 mmol/mol and ≤80 mmol/mol)]. Analysis was carried out according to intention-to-treat.
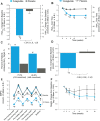
Results: At baseline, the mean participant age was 58 years; mean HbA1c was 8.4% (68 mmol/mol) and mean weight was 85.5 kg. Dulaglutide 1.5 mg was superior to placebo at 24 weeks for HbA1c reduction from baseline with a between-group HbA1c difference of -1.3% [95% confidence interval (CI) -1.6, -1.0] or -14 mmol/mol (95% CI -17, -11); p < 0.001. A greater proportion of participants in the dulaglutide group reached an HbA1c level of <7.0% (53 mmol/mol) compared with placebo (55.3% vs 18.9%; p < 0.001). Dulaglutide significantly decreased fasting serum glucose from baseline compared with placebo (between-group difference -1.86 mmol/l (95% CI -2.58, -1.14) or -33.54 mg/dl (95% CI -46.55, -20.53); p < 0.001. Weight was decreased significantly from baseline in the dulaglutide group (p < 0.001); the between-group difference was not significant. The most common treatment-emergent adverse events for dulaglutide 1.5 mg were gastrointestinal: nausea (10.5%), diarrhoea (8.4%) and eructation (5.9%). Total hypoglycaemia was higher with dulaglutide 1.5 mg vs placebo (2.37 and 0.07 events/participant/year, respectively; p = 0.025). No severe hypoglycaemia was reported.
Conclusions: Once-weekly dulaglutide 1.5 mg had a favourable benefit/risk profile when added to glimepiride monotherapy.
Keywords: dulaglutide; glucagon-like peptide-1; type 2 diabetes.
© 2016 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Figures
References
-
- Inzucchi SE, Bergenstal RM, Buse JB et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38: 140–149. - PubMed
-
- Jabbour S, Ziring B. Advantages of extended‐release metformin in patients with type 2 diabetes mellitus. Postgrad Med 2011; 123: 15–23. - PubMed
-
- Pongwecharak J, Tengmeesri N, Malanusorn N, Panthong M, Pawangkapin N. Prescribing metformin in type 2 diabetes with a contraindication: prevalence and outcome. Pharm World Sci 2009; 31: 481–486. - PubMed
-
- AHA . Heart Disease and Stroke Statistics: 2005 Update. Dallas: American Heart Association, 2005.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

