Decreased Brain Levels of Vitamin B12 in Aging, Autism and Schizophrenia
- PMID: 26799654
- PMCID: PMC4723262
- DOI: 10.1371/journal.pone.0146797
Decreased Brain Levels of Vitamin B12 in Aging, Autism and Schizophrenia
Abstract
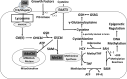
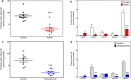
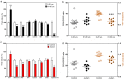
Many studies indicate a crucial role for the vitamin B12 and folate-dependent enzyme methionine synthase (MS) in brain development and function, but vitamin B12 status in the brain across the lifespan has not been previously investigated. Vitamin B12 (cobalamin, Cbl) exists in multiple forms, including methylcobalamin (MeCbl) and adenosylcobalamin (AdoCbl), serving as cofactors for MS and methylmalonylCoA mutase, respectively. We measured levels of five Cbl species in postmortem human frontal cortex of 43 control subjects, from 19 weeks of fetal development through 80 years of age, and 12 autistic and 9 schizophrenic subjects. Total Cbl was significantly lower in older control subjects (> 60 yrs of age), primarily reflecting a >10-fold age-dependent decline in the level of MeCbl. Levels of inactive cyanocobalamin (CNCbl) were remarkably higher in fetal brain samples. In both autistic and schizophrenic subjects MeCbl and AdoCbl levels were more than 3-fold lower than age-matched controls. In autistic subjects lower MeCbl was associated with decreased MS activity and elevated levels of its substrate homocysteine (HCY). Low levels of the antioxidant glutathione (GSH) have been linked to both autism and schizophrenia, and both total Cbl and MeCbl levels were decreased in glutamate-cysteine ligase modulatory subunit knockout (GCLM-KO) mice, which exhibit low GSH levels. Thus our findings reveal a previously unrecognized decrease in brain vitamin B12 status across the lifespan that may reflect an adaptation to increasing antioxidant demand, while accelerated deficits due to GSH deficiency may contribute to neurodevelopmental and neuropsychiatric disorders.
Conflict of interest statement
Figures



Similar articles
-
Decreased cortical Nrf2 gene expression in autism and its relationship to thiol and cobalamin status.Biochimie. 2022 Jan;192:1-12. doi: 10.1016/j.biochi.2021.09.006. Epub 2021 Sep 10. Biochimie. 2022. PMID: 34517051
-
Cobalamin coenzyme forms are not likely to be superior to cyano- and hydroxyl-cobalamin in prevention or treatment of cobalamin deficiency.Mol Nutr Food Res. 2015 Jul;59(7):1364-72. doi: 10.1002/mnfr.201500019. Epub 2015 May 12. Mol Nutr Food Res. 2015. PMID: 25820384 Free PMC article. Review.
-
Age-dependent decrease and alternative splicing of methionine synthase mRNA in human cerebral cortex and an accelerated decrease in autism.PLoS One. 2013;8(2):e56927. doi: 10.1371/journal.pone.0056927. Epub 2013 Feb 20. PLoS One. 2013. PMID: 23437274 Free PMC article.
-
Ethanol lowers glutathione in rat liver and brain and inhibits methionine synthase in a cobalamin-dependent manner.Alcohol Clin Exp Res. 2011 Feb;35(2):277-83. doi: 10.1111/j.1530-0277.2010.01343.x. Epub 2010 Dec 1. Alcohol Clin Exp Res. 2011. PMID: 21121936 Free PMC article.
-
Inherited errors of cobalamin metabolism and their management.Baillieres Clin Haematol. 1995 Sep;8(3):567-601. doi: 10.1016/s0950-3536(05)80221-5. Baillieres Clin Haematol. 1995. PMID: 8534962 Review.
Cited by
-
Differences in Tissue Distribution of Cyano⁻B12 and Hydroxo⁻B12 One Week after Oral Intake: An Experimental Study in Male Wistar Rats.Nutrients. 2018 Oct 12;10(10):1487. doi: 10.3390/nu10101487. Nutrients. 2018. PMID: 30322035 Free PMC article.
-
Role of vitamin B12 deficiency in ischemic stroke risk and outcome.Neural Regen Res. 2021 Mar;16(3):470-474. doi: 10.4103/1673-5374.291381. Neural Regen Res. 2021. PMID: 32985467 Free PMC article. Review.
-
Micronutrient-Associated Single Nucleotide Polymorphism and Mental Health: A Mendelian Randomization Study.Nutrients. 2024 Jun 27;16(13):2042. doi: 10.3390/nu16132042. Nutrients. 2024. PMID: 38999789 Free PMC article.
-
Regulation of Reactive Oxygen Species-Mediated Damage in the Pathogenesis of Schizophrenia.Brain Sci. 2020 Oct 16;10(10):742. doi: 10.3390/brainsci10100742. Brain Sci. 2020. PMID: 33081261 Free PMC article. Review.
-
Vitamin B12 Levels in Methamphetamine Addicts.Front Behav Neurosci. 2018 Dec 18;12:320. doi: 10.3389/fnbeh.2018.00320. eCollection 2018. Front Behav Neurosci. 2018. PMID: 30618670 Free PMC article.
References
-
- Sharma A, Kramer ML, Wick PF, Liu D, Chari S, Shim S. et al. D4 dopamine receptor-mediated phospholipid methylation and its implications for mental illnesses such as schizophrenia. Mol Psychiatry. 1999; 4:235–246. - PubMed
-
- Waly M, Olteanu H, Banerjee R, Choi SW, Mason JB, Parker BS, et al. Activation of methionine synthase by insulin-like growth factor-1 and dopamine: a target for neurodevelopmental toxins and thimerosal. Mol Psychiatry. 2004; 9:358–370. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

