Evaluation of cell-laden polyelectrolyte hydrogels incorporating poly(L-Lysine) for applications in cartilage tissue engineering
- PMID: 26799859
- PMCID: PMC4754156
- DOI: 10.1016/j.biomaterials.2016.01.020
Evaluation of cell-laden polyelectrolyte hydrogels incorporating poly(L-Lysine) for applications in cartilage tissue engineering
Abstract
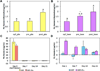
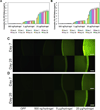
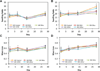
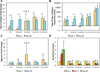
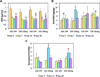
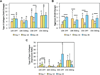
To address the lack of reliable long-term solutions for cartilage injuries, strategies in tissue engineering are beginning to leverage developmental processes to spur tissue regeneration. This study focuses on the use of poly(L-lysine) (PLL), previously shown to up-regulate mesenchymal condensation during developmental skeletogenesis in vitro, as an early chondrogenic stimulant of mesenchymal stem cells (MSCs). We characterized the effect of PLL incorporation on the swelling and degradation of oligo(poly(ethylene) glycol) fumarate) (OPF)-based hydrogels as functions of PLL molecular weight and dosage. Furthermore, we investigated the effect of PLL incorporation on the chondrogenic gene expression of hydrogel-encapsulated MSCs. The incorporation of PLL resulted in early enhancements of type II collagen and aggrecan gene expression and type II/type I collagen expression ratios when compared to blank controls. The presentation of PLL to MSCs encapsulated in OPF hydrogels also enhanced N-cadherin gene expression under certain culture conditions, suggesting that PLL may induce the expression of condensation markers in synthetic hydrogel systems. In summary, PLL can function as an inductive factor that primes the cellular microenvironment for early chondrogenic gene expression but may require additional biochemical factors for the generation of fully functional chondrocytes.
Keywords: Cartilage tissue engineering; Chondrogenic differentiation; Condensation; Hydrogel; Mesenchymal stem cells; Poly(l-lysine).
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures


References
-
- Behery O, Siston RA, Harris JD, Flanigan DC. Treatment of cartilage defects of the knee: expanding on the existing algorithm. Clinical journal of sport medicine : official journal of the Canadian Academy of Sport Medicine. 2014;24:21–30. - PubMed
-
- Cross M, Smith E, Hoy D, Carmona L, Wolfe F, Vos T, et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Annals of the rheumatic diseases. 2014;73:1316–1322. - PubMed
-
- Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Annals of the rheumatic diseases. 2014;73:1323–1330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

