MicroRNA-134 Contributes to Glucose-Induced Endothelial Cell Dysfunction and This Effect Can Be Reversed by Far-Infrared Irradiation
- PMID: 26799933
- PMCID: PMC4723308
- DOI: 10.1371/journal.pone.0147067
MicroRNA-134 Contributes to Glucose-Induced Endothelial Cell Dysfunction and This Effect Can Be Reversed by Far-Infrared Irradiation
Abstract
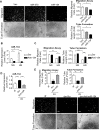
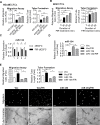
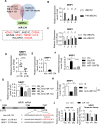
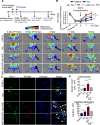
Diabetes mellitus (DM) is a metabolic disease that is increasing worldwide. Furthermore, it is associated with the deregulation of vascular-related functions, which can develop into major complications among DM patients. Endothelial colony forming cells (ECFCs) have the potential to bring about medical repairs because of their post-natal angiogenic activities; however, such activities are impaired by high glucose- (HG) and the DM-associated conditions. Far-infrared radiation (FIR) transfers energy as heat that is perceived by the thermoreceptors in human skin. Several studies have revealed that FIR improves vascular endothelial functioning and boost angiogenesis. FIR has been used as anti-inflammatory therapy and as a clinical treatment for peripheral circulation improvement. In addition to vascular repair, there is increasing evidence to show that FIR can be applied to a variety of diseases, including cardiovascular disorders, hypertension and arthritis. Yet mechanism of action of FIR and the biomarkers that indicate FIR effects remain unclear. MicroRNA-134 (miR-134-5p) was identified by small RNA sequencing as being increased in high glucose (HG) treated dfECFCs (HG-dfECFCs). Highly expressed miR-134 was also validated in dmECFCs by RT-qPCR and it is associated with impaired angiogenic activities of ECFCs. The functioning of ECFCs is improved by FIR treatment and this occurs via a reduction in the level of miR-134 and an increase in the NRIP1 transcript, a direct target of miR-134. Using a mouse ischemic hindlimb model, the recovery of impaired blood flow in the presence of HG-dfECFCs was improved by FIR pretreatment and this enhanced functionality was decreased when there was miR-134 overexpression in the FIR pretreated HG-dfECFCs. In conclusion, our results reveal that the deregulation of miR-134 is involved in angiogenic defects found in DM patients. FIR treatment improves the angiogenic activity of HG-dfECFCs and dmECFCs and FIR has potential as a treatment for DM. Detection of miR-134 expression in FIR-treated ECFCs should help us to explore further the effectiveness of FIR therapy.
Conflict of interest statement
Figures



References
-
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes care. 2004;27(5):1047–53. - PubMed
-
- Martin A, Komada MR, Sane DC. Abnormal angiogenesis in diabetes mellitus. Medicinal research reviews. 2003;23(2):117–45. - PubMed
-
- Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arteriosclerosis, thrombosis, and vascular biology. 2004;24(2):288–93. - PubMed
-
- Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104(9):2752–60. - PubMed
-
- Mukai N, Akahori T, Komaki M, Li Q, Kanayasu-Toyoda T, Ishii-Watabe A, et al. A comparison of the tube forming potentials of early and late endothelial progenitor cells. Experimental cell research. 2008;314(3):430–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

