Phosphate starvation: a novel signal that triggers ESX-5 secretion in Mycobacterium tuberculosis
- PMID: 26800324
- PMCID: PMC4863468
- DOI: 10.1111/mmi.13332
Phosphate starvation: a novel signal that triggers ESX-5 secretion in Mycobacterium tuberculosis
Abstract
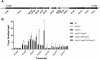
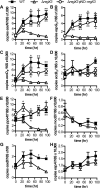
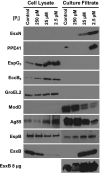
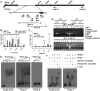
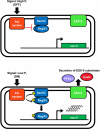
Mycobacterium tuberculosis uses the Type VII ESX secretion systems to transport proteins across its complex cell wall. ESX-5 has been implicated in M. tuberculosis virulence, but the regulatory mechanisms controlling ESX-5 secretion were unknown. Here we uncover a link between ESX-5 and the Pst/SenX3-RegX3 system that controls gene expression in response to phosphate availability. The DNA-binding response regulator RegX3 is normally activated by phosphate limitation. Deletion of pstA1, which encodes a Pst phosphate uptake system component, causes constitutive activation of RegX3. A ΔpstA1 mutant exhibited RegX3-dependent overexpression of esx-5 genes and hyper-secretion of the ESX-5 substrates EsxN and PPE41 when the bacteria were grown in phosphate-rich medium. In wild-type M. tuberculosis, phosphate limitation activated esx-5 transcription and secretion of both EsxN and PPE41, and this response required RegX3. Electrophoretic mobility shift assays revealed that RegX3 binds directly to a promoter within the esx-5 locus. Remarkably, phosphate limitation also induced secretion of EsxB, an effector of the virulence-associated ESX-1 secretion system, though this induction was RegX3 independent. Our work demonstrates that the Pst/SenX3-RegX3 system directly regulates ESX-5 secretion at the transcriptional level in response to phosphate availability and defines phosphate limitation as an environmental signal that activates ESX-5 secretion.
© 2016 John Wiley & Sons Ltd.
Figures


Similar articles
-
Mycobacterium tuberculosis PhoY Proteins Promote Persister Formation by Mediating Pst/SenX3-RegX3 Phosphate Sensing.mBio. 2017 Jul 11;8(4):e00494-17. doi: 10.1128/mBio.00494-17. mBio. 2017. PMID: 28698272 Free PMC article.
-
Mycobacterium tuberculosis Pst/SenX3-RegX3 Regulates Membrane Vesicle Production Independently of ESX-5 Activity.mBio. 2018 Jun 12;9(3):e00778-18. doi: 10.1128/mBio.00778-18. mBio. 2018. PMID: 29895636 Free PMC article.
-
Mycobacterium tuberculosis Requires Regulation of ESX-5 Secretion for Virulence in Irgm1-Deficient Mice.Infect Immun. 2019 Jan 24;87(2):e00660-18. doi: 10.1128/IAI.00660-18. Print 2019 Feb. Infect Immun. 2019. PMID: 30455198 Free PMC article.
-
Phosphate responsive regulation provides insights for ESX-5 function in Mycobacterium tuberculosis.Curr Genet. 2016 Nov;62(4):759-763. doi: 10.1007/s00294-016-0604-4. Epub 2016 Apr 22. Curr Genet. 2016. PMID: 27105642 Free PMC article. Review.
-
Modular Organization of the ESX-5 Secretion System in Mycobacterium tuberculosis.Front Cell Infect Microbiol. 2016 May 2;6:49. doi: 10.3389/fcimb.2016.00049. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27200304 Free PMC article. Review.
Cited by
-
Insights into the molecular determinants involved in Mycobacterium tuberculosis persistence and their therapeutic implications.Virulence. 2021 Dec;12(1):2721-2749. doi: 10.1080/21505594.2021.1990660. Virulence. 2021. PMID: 34637683 Free PMC article. Review.
-
Mycobacterium tuberculosis PhoY Proteins Promote Persister Formation by Mediating Pst/SenX3-RegX3 Phosphate Sensing.mBio. 2017 Jul 11;8(4):e00494-17. doi: 10.1128/mBio.00494-17. mBio. 2017. PMID: 28698272 Free PMC article.
-
Extracellular Vesicles in Mycobacteria and Tuberculosis.Front Cell Infect Microbiol. 2022 May 27;12:912831. doi: 10.3389/fcimb.2022.912831. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35719351 Free PMC article. Review.
-
Mycobacterium tuberculosis Pst/SenX3-RegX3 Regulates Membrane Vesicle Production Independently of ESX-5 Activity.mBio. 2018 Jun 12;9(3):e00778-18. doi: 10.1128/mBio.00778-18. mBio. 2018. PMID: 29895636 Free PMC article.
-
WhiB6 regulation of ESX-1 gene expression is controlled by a negative feedback loop in Mycobacterium marinum.Proc Natl Acad Sci U S A. 2017 Dec 12;114(50):E10772-E10781. doi: 10.1073/pnas.1710167114. Epub 2017 Nov 27. Proc Natl Acad Sci U S A. 2017. PMID: 29180415 Free PMC article.
References
-
- Abdallah AM, Bestebroer J, Savage NDL, de Punder K, van Zon M, Wilson L, Korbee CJ, van der Sar AM, Ottenhoff THM, van der Wel NN, Bitter W, Peters PJ. Mycobacterial secretion systems ESX-1 and ESX-5 play distinct roles in host cell death and inflammasome activation. J. Immunol. 2011;187:4744–4753. - PubMed
-
- Bottai D, Di Luca M, Majlessi L, Frigui W, Simeone R, Sayes F, Bitter W, Brennan MJ, Leclerc C, Batoni G, Campa M, Brosch R, Esin S. Disruption of the ESX-5 system of Mycobacterium tuberculosis causes loss of PPE protein secretion, reduction of cell wall integrity and strong attenuation. Mol. Microbiol. 2012;83:1195–1209. - PubMed
-
- Chen JM, Zhang M, Rybniker J, Boy-Röttger S, Dhar N, Pojer F, Cole ST. Mycobacterium tuberculosis EspB binds phospholipids and mediates EsxA-independent virulence. Mol. Microbiol. 2013;89:1154–1166. - PubMed
-
- Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CEI, Takala F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail MA, Rajandream MA, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston JE, Taylor K, Whitehead S, Barrell BG. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

