Novel α-L-Fucosidases from a Soil Metagenome for Production of Fucosylated Human Milk Oligosaccharides
- PMID: 26800369
- PMCID: PMC4723247
- DOI: 10.1371/journal.pone.0147438
Novel α-L-Fucosidases from a Soil Metagenome for Production of Fucosylated Human Milk Oligosaccharides
Abstract
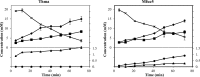
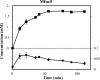
This paper describes the discovery of novel α-L-fucosidases and evaluation of their potential to catalyse the transglycosylation reaction leading to production of fucosylated human milk oligosaccharides. Seven novel α-L-fucosidase-encoding genes were identified by functional screening of a soil-derived metagenome library and expressed in E. coli as recombinant 6xHis-tagged proteins. All seven fucosidases belong to glycosyl hydrolase family 29 (GH 29). Six of the seven α-L-fucosidases were substrate-inhibited, moderately thermostable and most hydrolytically active in the pH range 6-7, when tested with para-nitrophenyl-α-L-fucopyranoside (pNP-Fuc) as the substrate. In contrast, one fucosidase (Mfuc6) exhibited a high pH optimum and an unusual sigmoidal kinetics towards pNP-Fuc substrate. When tested for trans-fucosylation activity using pNP-Fuc as donor, most of the enzymes were able to transfer fucose to pNP-Fuc (self-condensation) or to lactose. With the α-L-fucosidase from Thermotoga maritima and the metagenome-derived Mfuc5, different fucosyllactose variants including the principal fucosylated HMO 2'-fucosyllactose were synthesised in yields of up to ~6.4%. Mfuc5 was able to release fucose from xyloglucan and could also use it as a fucosyl-donor for synthesis of fucosyllactose. This is the first study describing the use of glycosyl hydrolases for the synthesis of genuine fucosylated human milk oligosaccharides.
Conflict of interest statement
Figures
Similar articles
-
Substrate specificity and transfucosylation activity of GH29 α-l-fucosidases for enzymatic production of human milk oligosaccharides.N Biotechnol. 2018 Mar 25;41:34-45. doi: 10.1016/j.nbt.2017.12.002. Epub 2017 Dec 6. N Biotechnol. 2018. PMID: 29221760
-
Infant Gut Microbial Metagenome Mining of α-l-Fucosidases with Activity on Fucosylated Human Milk Oligosaccharides and Glycoconjugates.Microbiol Spectr. 2022 Aug 31;10(4):e0177522. doi: 10.1128/spectrum.01775-22. Epub 2022 Aug 9. Microbiol Spectr. 2022. PMID: 35943155 Free PMC article.
-
Synthesis of fucosyllactose using α-L-fucosidases GH29 from infant gut microbial metagenome.Appl Microbiol Biotechnol. 2024 May 21;108(1):338. doi: 10.1007/s00253-024-13178-3. Appl Microbiol Biotechnol. 2024. PMID: 38771321 Free PMC article.
-
α-L-Fucosidases and their applications for the production of fucosylated human milk oligosaccharides.Appl Microbiol Biotechnol. 2020 Jul;104(13):5619-5631. doi: 10.1007/s00253-020-10635-7. Epub 2020 May 1. Appl Microbiol Biotechnol. 2020. PMID: 32356197 Review.
-
Enzymatic transfucosylation for synthesis of human milk oligosaccharides.Carbohydr Res. 2020 Jul;493:108029. doi: 10.1016/j.carres.2020.108029. Epub 2020 May 8. Carbohydr Res. 2020. PMID: 32445980 Review.
Cited by
-
Ancient origin of fucosylated xyloglucan in charophycean green algae.Commun Biol. 2021 Jun 17;4(1):754. doi: 10.1038/s42003-021-02277-w. Commun Biol. 2021. PMID: 34140625 Free PMC article.
-
Production and characterization of Aspergillus niger GH29 family α-fucosidase and production of a novel non-reducing 1-fucosyllactose.Glycoconj J. 2020 Apr;37(2):221-229. doi: 10.1007/s10719-019-09896-w. Epub 2019 Dec 2. Glycoconj J. 2020. PMID: 31792892 Free PMC article.
-
Architecture Insight of Bifidobacterial α-L-Fucosidases.Int J Mol Sci. 2021 Aug 6;22(16):8462. doi: 10.3390/ijms22168462. Int J Mol Sci. 2021. PMID: 34445166 Free PMC article.
-
Improved Transglycosylation by a Xyloglucan-Active α-l-Fucosidase from Fusarium graminearum.J Fungi (Basel). 2020 Nov 18;6(4):295. doi: 10.3390/jof6040295. J Fungi (Basel). 2020. PMID: 33217923 Free PMC article.
-
A Robust α-l-Fucosidase from Prevotella nigrescens for Glycoengineering Therapeutic Antibodies.ACS Chem Biol. 2024 Jul 19;19(7):1515-1524. doi: 10.1021/acschembio.4c00196. Epub 2024 Jun 24. ACS Chem Biol. 2024. PMID: 38912881 Free PMC article.
References
-
- Vanhooren PT, Vandamme EJ. L-Fucose: Occurrence, physiological role, chemical, enzymatic and microbial synthesis. J Chem Technol Biot. 1999;74: 479–497.
-
- Becker DJ, Lowe JB. Fucose: Biosynthesis and biological function in mammals. Glycobiology. 2003;13: 41R–53R. - PubMed
-
- Morrow AL, Ruiz-Palacios GM, Altaye M, Jiang X, Lourdes Guerrero M, Meinzen-Derr JK, et al. Human milk oligosaccharides are associated with protection against diarrhea in breast-fed infants. J Pediatr. 2004;145: 297–303. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

