Systematic and quantitative assessment of the effect of chronic kidney disease on CYP2D6 and CYP3A4/5
- PMID: 26800425
- PMCID: PMC5024330
- DOI: 10.1002/cpt.337
Systematic and quantitative assessment of the effect of chronic kidney disease on CYP2D6 and CYP3A4/5
Abstract
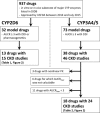
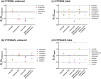
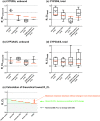
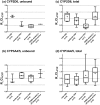
Recent reviews suggest that chronic kidney disease (CKD) can affect the pharmacokinetics of nonrenally eliminated drugs, but the impact of CKD on individual elimination pathways has not been systematically evaluated. In this study we developed a comprehensive dataset of the effect of CKD on the pharmacokinetics of CYP2D6- and CYP3A4/5-metabolized drugs. Drugs for evaluation were selected based on clinical drug-drug interaction (CYP3A4/5 and CYP2D6) and pharmacogenetic (CYP2D6) studies. Information from dedicated CKD studies was available for 13 and 18 of the CYP2D6 and CYP3A4/5 model drugs, respectively. Analysis of these data suggested that CYP2D6-mediated clearance is generally decreased in parallel with the severity of CKD. There was no apparent relationship between the severity of CKD and CYP3A4/5-mediated clearance. The observed elimination-route dependency in CKD effects between CYP2D6 and CYP3A4/5 may inform the need to conduct clinical CKD studies with nonrenally eliminated drugs for optimal use of drugs in patients with CKD.
© 2015, The American Society for Clinical Pharmacology and Therapeutics.
Figures
Similar articles
-
Effect of CKD and dialysis modality on exposure to drugs cleared by nonrenal mechanisms.Am J Kidney Dis. 2015 Apr;65(4):574-82. doi: 10.1053/j.ajkd.2014.09.015. Epub 2014 Nov 21. Am J Kidney Dis. 2015. PMID: 25453994
-
The Influence of the CYP3A4*22 Polymorphism and CYP2D6 Polymorphisms on Serum Concentrations of Aripiprazole, Haloperidol, Pimozide, and Risperidone in Psychiatric Patients.J Clin Psychopharmacol. 2015 Jun;35(3):228-36. doi: 10.1097/JCP.0000000000000319. J Clin Psychopharmacol. 2015. PMID: 25868121
-
Effect of Chronic Kidney Disease on Nonrenal Elimination Pathways: A Systematic Assessment of CYP1A2, CYP2C8, CYP2C9, CYP2C19, and OATP.Clin Pharmacol Ther. 2018 May;103(5):854-867. doi: 10.1002/cpt.807. Epub 2017 Oct 9. Clin Pharmacol Ther. 2018. PMID: 28990182 Free PMC article.
-
The Effects of CKD on Cytochrome P450-Mediated Drug Metabolism.Adv Chronic Kidney Dis. 2016 Mar;23(2):67-75. doi: 10.1053/j.ackd.2015.10.002. Adv Chronic Kidney Dis. 2016. PMID: 26979145 Review.
-
PharmVar GeneFocus: CYP2D6.Clin Pharmacol Ther. 2020 Jan;107(1):154-170. doi: 10.1002/cpt.1643. Epub 2019 Dec 9. Clin Pharmacol Ther. 2020. PMID: 31544239 Free PMC article. Review.
Cited by
-
Elucidating the Plasma and Liver Pharmacokinetics of Simeprevir in Special Populations Using Physiologically Based Pharmacokinetic Modelling.Clin Pharmacokinet. 2017 Jul;56(7):781-792. doi: 10.1007/s40262-016-0476-2. Clin Pharmacokinet. 2017. PMID: 27896690
-
Use of Physiologically Based Pharmacokinetic Modeling to Evaluate the Effect of Chronic Kidney Disease on the Disposition of Hepatic CYP2C8 and OATP1B Drug Substrates.Clin Pharmacol Ther. 2019 Mar;105(3):719-729. doi: 10.1002/cpt.1205. Epub 2018 Oct 26. Clin Pharmacol Ther. 2019. PMID: 30074626 Free PMC article.
-
Modeling Drug Disposition and Drug-Drug Interactions Through Hypothesis-Driven Physiologically Based Pharmacokinetics: a Reversal Translation Perspective.Eur J Drug Metab Pharmacokinet. 2018 Jun;43(3):369-371. doi: 10.1007/s13318-017-0452-8. Eur J Drug Metab Pharmacokinet. 2018. PMID: 29204788 No abstract available.
-
Reverse Translation in PBPK and QSP: Going Backwards in Order to Go Forward With Confidence.Clin Pharmacol Ther. 2018 Feb;103(2):224-232. doi: 10.1002/cpt.904. Epub 2017 Nov 9. Clin Pharmacol Ther. 2018. PMID: 29023678 Free PMC article. Review.
-
Inflammation Decreases Ciclosporin Metabolism in Allogeneic Hematopoietic Stem Cell Transplantation Recipients.J Clin Pharmacol. 2025 Mar;65(3):328-339. doi: 10.1002/jcph.6141. Epub 2024 Oct 9. J Clin Pharmacol. 2025. PMID: 39382849 Free PMC article.
References
-
- Huang, S.M. & Temple, R. Is this the drug or dose for you? Impact and consideration of ethnic factors in global drug development, regulatory review, and clinical practice. Clin. Pharmacol. Ther. 84, 287–294 (2008). - PubMed
-
- US Department of Health and Human Services , Food and Drug Administration , Center for Drug Evaluation and Research (CDER) . Draft Guidance / Guidance for Industry. Pharmacokinetics in Patients with Impaired Renal Function — Study Design, Data Analysis, and Impact on Dosing and Labeling. <http://www.fda.gov/downloads/Drugs/Guidances/UCM204959.pdf, http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidanc...> (2010). Accessed 26 March, 2015.
-
- European Medicines Agency . Draft guideline on the evaluation of the pharmacokinetics of medicinal products in patients with decreased renal function. <http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin...> (2014). Accessed 26 March, 2015.
-
- Nolin, T.D. , Naud, J. , Leblond, F.A. & Pichette, V. Emerging evidence of the impact of kidney disease on drug metabolism and transport. Clin. Pharmacol. Ther. 83, 898–903 (2008). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

