Factor XI Deficiency Protects Against Atherogenesis in Apolipoprotein E/Factor XI Double Knockout Mice
- PMID: 26800563
- PMCID: PMC4785893
- DOI: 10.1161/ATVBAHA.115.306954
Factor XI Deficiency Protects Against Atherogenesis in Apolipoprotein E/Factor XI Double Knockout Mice
Abstract
Objective: Atherosclerosis and atherothrombosis are still major causes of mortality in the Western world, even after the widespread use of cholesterol-lowering medications. Recently, an association between local thrombin generation and atherosclerotic burden has been reported. Here, we studied the role of factor XI (FXI) deficiency in the process of atherosclerosis in mice.
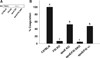
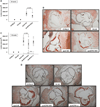
Approach and results: Apolipoprotein E/FXI double knockout mice, created for the first time in our laboratory. There was no difference in cholesterol levels or lipoprotein profiles between apolipoprotein E knockout and double knockout mice. Nevertheless, in 24-week-old double knockout mice, the atherosclerotic lesion area in the aortic sinus was reduced by 32% (P=0.004) in comparison with apolipoprotein E knockout mice. In 42-week-old double knockout mice, FXI deficiency inhibited atherosclerosis progression significantly in the aortic sinus (25% reduction, P=0.024) and in the aortic arch (49% reduction, P=0.028), with a prominent reduction of macrophage infiltration in the atherosclerotic lesions.
Conclusions: FXI deprivation was shown to slow down atherogenesis in mice. The results suggest that the development of atherosclerosis can be prevented by targeting FXI.
Keywords: atherosclerosis; factor XI.
© 2016 American Heart Association, Inc.
Figures
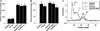



Comment in
-
The Clot Thickens in Atherosclerosis.Arterioscler Thromb Vasc Biol. 2016 Mar;36(3):425-6. doi: 10.1161/ATVBAHA.116.307094. Arterioscler Thromb Vasc Biol. 2016. PMID: 26912742 Free PMC article. No abstract available.
References
-
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. - PubMed
-
- Mastenbroek TG, van Geffen JP, Heemskerk JW, Cosemans JM. Acute and persistent platelet and coagulant activities in atherothrombosis. J Thromb Haemost. 2015;13(suppl 1):S272–S280. - PubMed
-
- Borissoff JI, Heeneman S, Kilinç E, Kassák P, Van Oerle R, Winckers K, Govers-Riemslag JW, Hamulyák K, Hackeng TM, Daemen MJ, ten Cate H, Spronk HM. Early atherosclerosis exhibits an enhanced procoagulant state. Circulation. 2010;122:821–830. - PubMed
-
- Loeffen R, Spronk HM, ten Cate H. The impact of blood coagulability on atherosclerosis and cardiovascular disease. J Thromb Haemost. 2012;10:1207–1216. - PubMed
-
- Borissoff JI, Spronk HM, ten Cate H. The hemostatic system as a modulator of atherosclerosis. N Engl J Med. 2011;364:1746–1760. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

