Discoidin Domain Receptor-1 Regulates Calcific Extracellular Vesicle Release in Vascular Smooth Muscle Cell Fibrocalcific Response via Transforming Growth Factor-β Signaling
- PMID: 26800565
- PMCID: PMC4767541
- DOI: 10.1161/ATVBAHA.115.307009
Discoidin Domain Receptor-1 Regulates Calcific Extracellular Vesicle Release in Vascular Smooth Muscle Cell Fibrocalcific Response via Transforming Growth Factor-β Signaling
Abstract
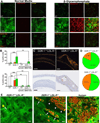
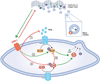
Objective: Collagen accumulation and calcification are major determinants of atherosclerotic plaque stability. Extracellular vesicle (EV)-derived microcalcifications in the collagen-poor fibrous cap may promote plaque rupture. In this study, we hypothesize that the collagen receptor discoidin domain receptor-1 (DDR-1) regulates collagen deposition and release of calcifying EVs by vascular smooth muscle cells (SMCs) through the transforming growth factor-β (TGF-β) pathway.
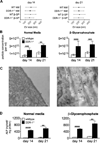
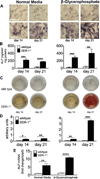
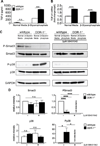
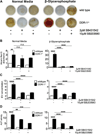
Approach and results: SMCs from the carotid arteries of DDR-1(-/-) mice and wild-type littermates (n=5-10 per group) were cultured in normal or calcifying media. At days 14 and 21, SMCs were harvested and EVs isolated for analysis. Compared with wild-type, DDR-1(-/-) SMCs exhibited a 4-fold increase in EV release (P<0.001) with concomitantly elevated alkaline phosphatase activity (P<0.0001) as a hallmark of EV calcifying potential. The DDR-1(-/-) phenotype was characterized by increased mineralization (Alizarin Red S and Osteosense, P<0.001 and P=0.002, respectively) and amorphous collagen deposition (P<0.001). We further identified a novel link between DDR-1 and the TGF-β pathway previously implicated in both fibrotic and calcific responses. An increase in TGF-β1 release by DDR-1(-/-) SMCs in calcifying media (P<0.001) stimulated p38 phosphorylation (P=0.02) and suppressed activation of Smad3. Inhibition of either TGF-β receptor-I or phospho-p38 reversed the fibrocalcific DDR-1(-/-) phenotype, corroborating a causal relationship between DDR-1 and TGF-β in EV-mediated vascular calcification.
Conclusions: DDR-1 interacts with the TGF-β pathway to restrict calcifying EV-mediated mineralization and fibrosis by SMCs. We therefore establish a novel mechanism of cell-matrix homeostasis in atherosclerotic plaque formation.
Keywords: extracellular matrix; extracellular vesicles; fibrosis; smooth muscle; transforming growth factors.
© 2016 American Heart Association, Inc.
Figures
References
-
- Maldonado N, Kelly-Arnold A, Vengrenyuk Y, Laudier D, Fallon JT, Virmani R, Cardoso L, Weinbaum S. A mechanistic analysis of the role of microcalcifications in atherosclerotic plaque stability: Potential implications for plaque rupture. American journal of physiology. Heart and circulatory physiology. 2012;303:H619–H628. - PMC - PubMed
-
- Vengrenyuk Y, Carlier S, Xanthos S, Cardoso L, Ganatos P, Virmani R, Einav S, Gilchrist L, Weinbaum S. A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:14678–14683. - PMC - PubMed
-
- Kapustin AN, Chatrou ML, Drozdov I, Zheng Y, Davidson SM, Soong D, Furmanik M, Sanchis P, De Rosales RT, Alvarez-Hernandez D, Shroff R, Yin X, Muller K, Skepper JN, Mayr M, Reutelingsperger CP, Chester A, Bertazzo S, Schurgers LJ, Shanahan CM. Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circ Res. 2015;116:1312–1323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

