Rice endosperm is cost-effective for the production of recombinant griffithsin with potent activity against HIV
- PMID: 26800650
- PMCID: PMC4865440
- DOI: 10.1111/pbi.12507
Rice endosperm is cost-effective for the production of recombinant griffithsin with potent activity against HIV
Abstract
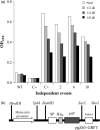
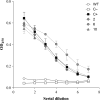
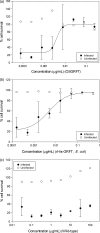
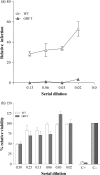
Protein microbicides containing neutralizing antibodies and antiviral lectins may help to reduce the rate of infection with human immunodeficiency virus (HIV) if it is possible to manufacture the components in large quantities at a cost affordable in HIV-endemic regions such as sub-Saharan Africa. We expressed the antiviral lectin griffithsin (GRFT), which shows potent neutralizing activity against HIV, in the endosperm of transgenic rice plants (Oryza sativa), to determine whether rice can be used to produce inexpensive GRFT as a microbicide ingredient. The yield of (OS) GRFT in the best-performing plants was 223 μg/g dry seed weight. We also established a one-step purification protocol, achieving a recovery of 74% and a purity of 80%, which potentially could be developed into a larger-scale process to facilitate inexpensive downstream processing. (OS) GRFT bound to HIV glycans with similar efficiency to GRFT produced in Escherichia coli. Whole-cell assays using purified (OS) GRFT and infectivity assays using crude extracts of transgenic rice endosperm confirmed that both crude and pure (OS) GRFT showed potent activity against HIV and the crude extracts were not toxic towards human cell lines, suggesting they could be administered as a microbicide with only minimal processing. A freedom-to-operate analysis confirmed that GRFT produced in rice is suitable for commercial development, and an economic evaluation suggested that 1.8 kg/ha of pure GRFT could be produced from rice seeds. Our data therefore indicate that rice could be developed as an inexpensive production platform for GRFT as a microbicide component.
Keywords: HIV; endosperm; griffithsin; lectin; microbicides; rice.
© 2016 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Figures


Similar articles
-
Unexpected synergistic HIV neutralization by a triple microbicide produced in rice endosperm.Proc Natl Acad Sci U S A. 2018 Aug 14;115(33):E7854-E7862. doi: 10.1073/pnas.1806022115. Epub 2018 Jul 30. Proc Natl Acad Sci U S A. 2018. PMID: 30061386 Free PMC article.
-
Cyanovirin-N produced in rice endosperm offers effective pre-exposure prophylaxis against HIV-1BaL infection in vitro.Plant Cell Rep. 2016 Jun;35(6):1309-19. doi: 10.1007/s00299-016-1963-5. Epub 2016 Mar 23. Plant Cell Rep. 2016. PMID: 27007716 Free PMC article.
-
Improving the large scale purification of the HIV microbicide, griffithsin.BMC Biotechnol. 2015 Feb 22;15(1):12. doi: 10.1186/s12896-015-0120-5. BMC Biotechnol. 2015. PMID: 25887919 Free PMC article.
-
Molecular farming using transgenic rice endosperm.Trends Biotechnol. 2022 Oct;40(10):1248-1260. doi: 10.1016/j.tibtech.2022.04.002. Epub 2022 May 10. Trends Biotechnol. 2022. PMID: 35562237 Review.
-
Griffithsin: An Antiviral Lectin with Outstanding Therapeutic Potential.Viruses. 2016 Oct 24;8(10):296. doi: 10.3390/v8100296. Viruses. 2016. PMID: 27783038 Free PMC article. Review.
Cited by
-
COVID-19 Crisis: How Can Plant Biotechnology Help?Plants (Basel). 2021 Feb 12;10(2):352. doi: 10.3390/plants10020352. Plants (Basel). 2021. PMID: 33673316 Free PMC article.
-
High-level expression of the HIV entry inhibitor griffithsin from the plastid genome and retention of biological activity in dried tobacco leaves.Plant Mol Biol. 2018 Jul;97(4-5):357-370. doi: 10.1007/s11103-018-0744-7. Epub 2018 Jun 9. Plant Mol Biol. 2018. PMID: 29948657 Free PMC article.
-
Generation of multi-layered protein bodies in N. benthamiana for the encapsulation of vaccine antigens.Front Plant Sci. 2023 Jan 17;14:1109270. doi: 10.3389/fpls.2023.1109270. eCollection 2023. Front Plant Sci. 2023. PMID: 36733717 Free PMC article.
-
Multiple gene expression in plants using MIDAS-P, a versatile type II restriction-based modular expression vector.Biotechnol Bioeng. 2022 Jun;119(6):1660-1672. doi: 10.1002/bit.28073. Epub 2022 Mar 16. Biotechnol Bioeng. 2022. PMID: 35238400 Free PMC article.
-
In Preparation for Outdoor Pharming: Griffithsin Can Be Expressed in Nicotiana excelsiana and Retains Activity After Storage as Silage.Front Bioeng Biotechnol. 2020 Mar 18;8:199. doi: 10.3389/fbioe.2020.00199. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32258012 Free PMC article.
References
-
- Arfi, Z.A. , Hellwig, S. , Drossard, J. , Fischer, R. and Buyel, J.F. (2015) Polyclonal antibodies for the specific detection of tobacco host cell proteins can be generated more efficiently following RuBisCO depletion and the removal of endotoxins. Biochem. J. (in press). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

