Characterization of a cold-active esterase from Serratia sp. and improvement of thermostability by directed evolution
- PMID: 26800680
- PMCID: PMC4722774
- DOI: 10.1186/s12896-016-0235-3
Characterization of a cold-active esterase from Serratia sp. and improvement of thermostability by directed evolution
Abstract
Background: In recent years, cold-active esterases have received increased attention due to their attractive properties for some industrial applications such as high catalytic activity at low temperatures.
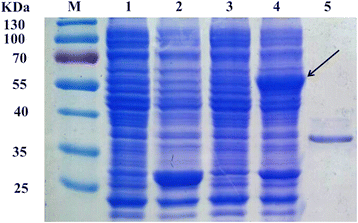
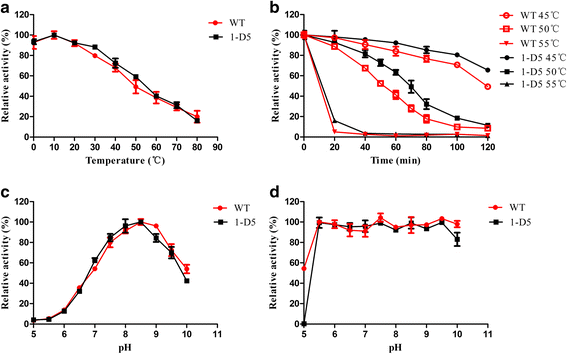
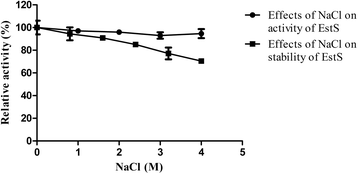
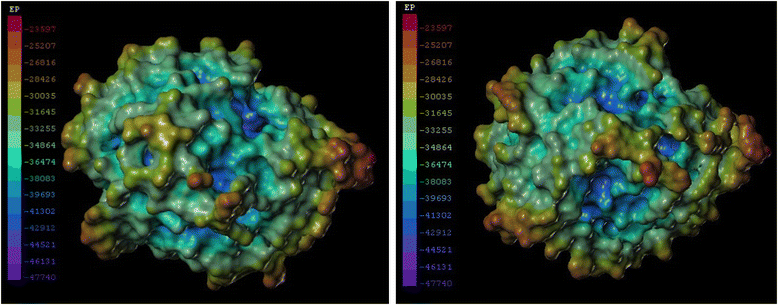
Results: An esterase-encoding gene (estS, 909 bp) from Serratia sp. was identified, cloned and expressed in Escherichia coli DE3 (BL21). The estS encoded a protein (EstS) of 302 amino acids with a predicted molecular weight of 32.5 kDa. It showed the highest activity at 10 °C and pH 8.5. EstS was cold active and retained ~92 % of its original activity at 0 °C. Thermal inactivation analysis showed that the T1/2 value of EstS was 50 min at 50 °C (residual activity 41.23 %) after 1 h incubation. EstS is also quite stable in high salt conditions and displayed better catalytic activity in the presence of 4 M NaCl. To improve the thermo-stability of EstS, variants of estS gene were created by error-prone PCR. A mutant 1-D5 (A43V, R116W, D147N) that showed higher thermo-stability than its wild type predecessor was selected. 1-D5 showed enhanced T1/2 of 70 min at 50 °C and retained 63.29 % of activity after incubation at 50 °C for 60 min, which were about 22 % higher than the wild type (WT). CD spectrum showed that the secondary structure of WT and 1-D5 are more or less similar, but an increase in β-sheets was recorded, which enhanced the thermostability of mutant protein.
Conclusion: EstS was a novel cold-active and salt-tolerant esterase and half-life of mutant 1-D5 was enhanced by 1.4 times compared with WT. The features of EstS are interesting and can be exploited for commercial applications. The results have also provided useful information about the structure and function of Est protein.
Figures




Similar articles
-
Identification and characterization of a novel salt-tolerant esterase from a Tibetan glacier metagenomic library.Biotechnol Prog. 2015 Jul-Aug;31(4):890-9. doi: 10.1002/btpr.2096. Epub 2015 May 15. Biotechnol Prog. 2015. PMID: 25920073
-
Characterization of a novel cold active and salt tolerant esterase from Zunongwangia profunda.Enzyme Microb Technol. 2016 Apr;85:1-11. doi: 10.1016/j.enzmictec.2015.12.013. Epub 2016 Jan 4. Enzyme Microb Technol. 2016. PMID: 26920474
-
Engineering and characterization of a novel low temperature active and thermo stable esterase from marine Enterobacter cloacae.Int J Biol Macromol. 2018 Oct 15;118(Pt A):304-310. doi: 10.1016/j.ijbiomac.2018.05.193. Epub 2018 May 26. Int J Biol Macromol. 2018. PMID: 29842953
-
Improved thermostability of esterase from Aspergillus fumigatus by site-directed mutagenesis.Enzyme Microb Technol. 2014 Oct;64-65:11-6. doi: 10.1016/j.enzmictec.2014.06.003. Epub 2014 Jun 28. Enzyme Microb Technol. 2014. PMID: 25152411
-
Molecular cloning and characterization of a novel cold-adapted family VIII esterase from a biogas slurry metagenomic library.J Microbiol Biotechnol. 2014 Nov 28;24(11):1484-9. doi: 10.4014/jmb.1406.06071. J Microbiol Biotechnol. 2014. PMID: 25394508
Cited by
-
Formation of recombinant bifunctional fusion protein: A newer approach to combine the activities of two enzymes in a single protein.PLoS One. 2022 Apr 1;17(4):e0265969. doi: 10.1371/journal.pone.0265969. eCollection 2022. PLoS One. 2022. Retraction in: PLoS One. 2022 Aug 31;17(8):e0273534. doi: 10.1371/journal.pone.0273534. PMID: 35363796 Free PMC article. Retracted.
-
Challenges and Recent Advances in Enzyme-Mediated Wastewater Remediation-A Review.Nanomaterials (Basel). 2021 Nov 19;11(11):3124. doi: 10.3390/nano11113124. Nanomaterials (Basel). 2021. PMID: 34835887 Free PMC article. Review.
-
Identification and Biochemical Characterization of a Novel Hormone-Sensitive Lipase Family Esterase Est19 from the Antarctic Bacterium Pseudomonas sp. E2-15.Biomolecules. 2021 Oct 20;11(11):1552. doi: 10.3390/biom11111552. Biomolecules. 2021. PMID: 34827549 Free PMC article.
-
Integrated (Meta) Genomic and Synthetic Biology Approaches to Develop New Biocatalysts.Mar Drugs. 2016 Mar 21;14(3):62. doi: 10.3390/md14030062. Mar Drugs. 2016. PMID: 27007381 Free PMC article. Review.
-
Molecular study on recombinant cold-adapted, detergent- and alkali stable esterase (EstRag) from Lysinibacillus sp.: a member of family VI.World J Microbiol Biotechnol. 2022 Sep 7;38(12):217. doi: 10.1007/s11274-022-03402-5. World J Microbiol Biotechnol. 2022. PMID: 36070019 Free PMC article.
References
-
- Li XL, Zhang WH, Wang YD, Dai YJ, Zhang HT, Wang Y, et al. A high-detergent-performance, cold-adapted lipase from Pseudomonas stutzeri PS59 suitable for detergent formulation. J Mol Catalysis B Enzymatic. 2014;102:16–24. doi: 10.1016/j.molcatb.2014.01.006. - DOI
-
- Tutino ML, Parrilli E, De Santi C, Giuliani M, Marino G, de Pascale D. Cold-adapted esterases and lipases: a biodiversity still under-exploited. Curr Chem Biol. 2010;4(1):74–83.
-
- Suzuki T, Nakayama T, Choo DW, Hirano Y, Kurihara T, Nishino T, et al. Cloning, heterologous expression, renaturation, and characterization of a cold-adapted esterase with unique primary structure from a psychrotroph Pseudomonas sp. strain B11-1. Protein Expr Purif. 2003;30(2):171–8. doi: 10.1016/S1046-5928(03)00128-1. - DOI - PubMed
-
- Kulakova L, Galkin A, Nakayama T, Nishino T, Esaki N. Cold-active esterase from Psychrobacter sp. Ant300: gene cloning, characterization, and the effects of Gly → Pro substitution near the active site on its catalytic activity and stability. Biochim Biophys Acta Protein Proteomics. 2004;1696(1):59–65. doi: 10.1016/j.bbapap.2003.09.008. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

