Inhibitory effects of Cheongsangbangpoong-tang on both inflammatory acne lesions and facial heat in patients with acne vulgaris: A randomized controlled trial protocol
- PMID: 26800893
- PMCID: PMC4722773
- DOI: 10.1186/s12906-016-1000-9
Inhibitory effects of Cheongsangbangpoong-tang on both inflammatory acne lesions and facial heat in patients with acne vulgaris: A randomized controlled trial protocol
Abstract
Background: Due to increasing interest from acne patients concerned about the side effects associated with conventional therapies, complementary and alternative medicine (CAM) has been suggested as a new therapeutic modality for acne vulgaris. Herbal medicine is one of these CAM treatments. Cheongsangbangpoong-tang (CBT) is a common herbal formula used in patients with acne vulgaris in the clinical practice of Korean Medicine (KM). However, despite the common use of CBT in clinical practice, the current level of evidence is insufficient to support an inhibitory effect of CBT on inflammatory acne lesions and facial heat. Therefore, this study was designed to assess the inhibitory effect of CBT on both inflammatory acne lesions and facial heat.
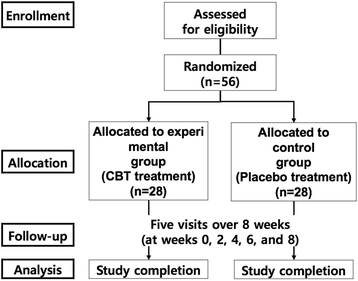
Methods/design: A randomized, double-blind, parallel-group, and placebo-controlled trial will be conducted. Fifty-six participants with acne vulgaris will be randomized into one of two groups: the CBT or placebo groups. After randomization, participants will be prescribed either CBT or placebo three times a day at a dose of 5 g after meals for 8 weeks. The following outcome measurements will be used in the examination of subjects: the mean percentage change and the count change of the inflammatory and non-inflammatory acne lesions, the temperature of facial points on digital infrared thermal imaging (DITI), serum cortisol, serum dehydroepiandrosterone-sulfate (DHEA-S), visual analogue scale (VAS), investigator global assessment (IGA), and severity score on the Korean Acne Grading System (KAGS) from baseline to the end of the trial.
Discussion: This trial will provide evidence regarding the inhibitory effect of CBT on inflammatory acne lesions and facial heat. The findings of this trial may have important implications for the more widespread use of CBT for the treatment of acne vulgaris.
Trial registration: The trial is registered with the Clinical Research Information Service (CRiS), Republic of Korea: KCT0001468 .
References
-
- Jeong W, Hong E, Shin J, Kim Y, Nam H, Lee J, Kim K. A Study on the Major Symptoms by Each Pattern of Acne Vulgaris. J Korean Med Ophthalmol Otolaryngol Dermatol. 2014;27(4):76–86. doi: 10.6114/jkood.2014.27.4.076. - DOI
-
- Shin J, Jeong W, Moon Y, Nam H, Kim Y, Lee J, Kim K. An Expert Survey for Developing the Pattern Diagnosis Instrument of Acne. J Korean Med Ophthalmol Otolaryngol Dermatol. 2015;28(2):23–32. doi: 10.6114/jkood.2015.28.2.023. - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous