Unique and Overlapping Functions of Formins Frl and DAAM During Ommatidial Rotation and Neuronal Development in Drosophila
- PMID: 26801180
- PMCID: PMC4788114
- DOI: 10.1534/genetics.115.181438
Unique and Overlapping Functions of Formins Frl and DAAM During Ommatidial Rotation and Neuronal Development in Drosophila
Abstract
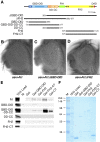
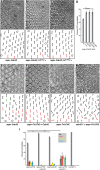
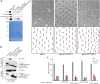
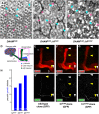
The noncanonical Frizzled/planar cell polarity (PCP) pathway regulates establishment of polarity within the plane of an epithelium to generate diversity of cell fates, asymmetric, but highly aligned structures, or to orchestrate the directional migration of cells during convergent extension during vertebrate gastrulation. In Drosophila, PCP signaling is essential to orient actin wing hairs and to align ommatidia in the eye, in part by coordinating the movement of groups of photoreceptor cells during ommatidial rotation. Importantly, the coordination of PCP signaling with changes in the cytoskeleton is essential for proper epithelial polarity. Formins polymerize linear actin filaments and are key regulators of the actin cytoskeleton. Here, we show that the diaphanous-related formin, Frl, the single fly member of the FMNL (formin related in leukocytes/formin-like) formin subfamily affects ommatidial rotation in the Drosophila eye and is controlled by the Rho family GTPase Cdc42. Interestingly, we also found that frl mutants exhibit an axon growth phenotype in the mushroom body, a center for olfactory learning in the Drosophila brain, which is also affected in a subset of PCP genes. Significantly, Frl cooperates with Cdc42 and another formin, DAAM, during mushroom body formation. This study thus suggests that different formins can cooperate or act independently in distinct tissues, likely integrating various signaling inputs with the regulation of the cytoskeleton. It furthermore highlights the importance and complexity of formin-dependent cytoskeletal regulation in multiple organs and developmental contexts.
Keywords: cytoskeleton; formin; neural development; noncanonical Wnt signaling; planar cell polarity.
Copyright © 2016 by the Genetics Society of America.
Figures




Similar articles
-
Molecular Dissection of DAAM Function during Axon Growth in Drosophila Embryonic Neurons.Cells. 2022 Apr 28;11(9):1487. doi: 10.3390/cells11091487. Cells. 2022. PMID: 35563792 Free PMC article.
-
The Formin DAAM Functions as Molecular Effector of the Planar Cell Polarity Pathway during Axonal Development in Drosophila.J Neurosci. 2015 Jul 15;35(28):10154-67. doi: 10.1523/JNEUROSCI.3708-14.2015. J Neurosci. 2015. PMID: 26180192 Free PMC article.
-
Planar cell polarity signaling in the Drosophila eye.Curr Top Dev Biol. 2010;93:189-227. doi: 10.1016/B978-0-12-385044-7.00007-2. Curr Top Dev Biol. 2010. PMID: 20959167 Free PMC article. Review.
-
Formin-dependent synaptic growth: evidence that Dlar signals via Diaphanous to modulate synaptic actin and dynamic pioneer microtubules.J Neurosci. 2008 Oct 29;28(44):11111-23. doi: 10.1523/JNEUROSCI.0833-08.2008. J Neurosci. 2008. PMID: 18971454 Free PMC article.
-
Formins as effector proteins of Rho GTPases.Small GTPases. 2014;5:e29513. doi: 10.4161/sgtp.29513. Epub 2014 Jun 10. Small GTPases. 2014. PMID: 24914801 Free PMC article. Review.
Cited by
-
The making of the Drosophila mushroom body.Front Physiol. 2023 Jan 13;14:1091248. doi: 10.3389/fphys.2023.1091248. eCollection 2023. Front Physiol. 2023. PMID: 36711013 Free PMC article. Review.
-
FRL and DAAM are required for lateral adhesion of interommatidial cells and patterning of the retinal floor.Development. 2023 Nov 15;150(22):dev201713. doi: 10.1242/dev.201713. Epub 2023 Nov 23. Development. 2023. PMID: 37997920 Free PMC article.
-
Overexpression of Drosophila NUAK or Constitutively-Active Formin-Like Promotes the Formation of Aberrant Myofibrils.Cytoskeleton (Hoboken). 2025 Jan 29:10.1002/cm.21999. doi: 10.1002/cm.21999. Online ahead of print. Cytoskeleton (Hoboken). 2025. PMID: 39876757
-
DAAM mediates the assembly of long-lived, treadmilling stress fibers in collectively migrating epithelial cells in Drosophila.Elife. 2021 Nov 23;10:e72881. doi: 10.7554/eLife.72881. Elife. 2021. PMID: 34812144 Free PMC article.
-
Molecular Dissection of DAAM Function during Axon Growth in Drosophila Embryonic Neurons.Cells. 2022 Apr 28;11(9):1487. doi: 10.3390/cells11091487. Cells. 2022. PMID: 35563792 Free PMC article.
References
-
- Basler K., Hafen E., 1990. Receptor tyrosine kinases mediate cell-cell interactions during Drosophila development. Prog. Growth Factor Res. 2: 15–27. - PubMed
-
- Basler K., Christen B., Hafen E., 1991. Ligand-independent activation of the sevenless receptor tyrosine kinase changes the fate of cells in the developing Drosophila eye. Cell 64: 1069–1081. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

