Validation of a Rapid and Sensitive UPLC-MS-MS Method Coupled with Protein Precipitation for the Simultaneous Determination of Seven Pyrethroids in 100 µL of Rat Plasma by Using Ammonium Adduct as Precursor Ion
- PMID: 26801239
- PMCID: PMC4885924
- DOI: 10.1093/jat/bkw002
Validation of a Rapid and Sensitive UPLC-MS-MS Method Coupled with Protein Precipitation for the Simultaneous Determination of Seven Pyrethroids in 100 µL of Rat Plasma by Using Ammonium Adduct as Precursor Ion
Abstract
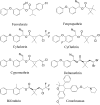
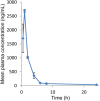
United States Environmental Protection Agency has recommended estimating pyrethroids' risk using cumulative exposure. For cumulative risk assessment, it would be useful to have a bioanalytical method for quantification of one or several pyrethroids simultaneously in a small sample volume to support toxicokinetic studies. Therefore, in the present study, a simple, sensitive and high-throughput ultraperformance liquid chromatography-tandem mass spectrometry method was developed and validated for simultaneous analysis of seven pyrethroids (fenvalerate, fenpropathrin, bifenthrin, lambda-cyhalothrin, cyfluthrin, cypermethrin and deltamethrin) in 100 µL of rat plasma. A simple single-step protein precipitation method was used for the extraction of target compounds. The total chromatographic run time of the method was 5 min. The chromatographic system used a Supelco C18 column and isocratic elution with a mobile phase consisting of methanol and 5 mM ammonium formate in the ratio of 90 : 10 (v/v). Mass spectrometer (API 4000) was operated in multiple reaction monitoring positive-ion mode using the electrospray ionization technique. The calibration curves were linear in the range of 7.8-2,000 ng/mL with correlation coefficients of ≥ 0.99. All validation parameters such as precision, accuracy, recovery, matrix effect and stability met the acceptance criteria according to the regulatory guidelines. The method was successfully applied to the toxicokinetic study of cypermethrin in rats. To the best of our knowledge, this is the first LC-MS-MS method for the simultaneous analysis of pyrethroids in rat plasma. This validated method with minimal modification can also be utilized for forensic and clinical toxicological applications due to its simplicity, sensitivity and rapidity.
© The Author 2016. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

References
-
- Adamis Z., Antal A., Fuzesi I., Molnar J., Nagy L., Susan M. (1985) Occupational exposure to organophosphorus insecticides and synthetic pyrethroid. International Archives of Occupational and Environmental Health, 56, 299–305. - PubMed
-
- Aldridge W.N. (1990) An assessment of the toxicological properties of pyrethroids and their neurotoxicity. Critical Reviews in Toxicology, 21, 89–104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

