Maternal high-fat diet impairs cardiac function in offspring of diabetic pregnancy through metabolic stress and mitochondrial dysfunction
- PMID: 26801311
- PMCID: PMC4867345
- DOI: 10.1152/ajpheart.00795.2015
Maternal high-fat diet impairs cardiac function in offspring of diabetic pregnancy through metabolic stress and mitochondrial dysfunction
Abstract
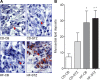
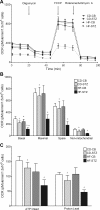
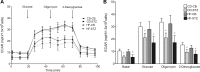
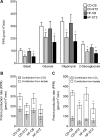
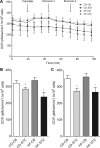
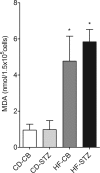
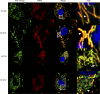
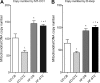
Offspring of diabetic pregnancies are at risk of cardiovascular disease at birth and throughout life, purportedly through fuel-mediated influences on the developing heart. Preventative measures focus on glycemic control, but the contribution of additional offenders, including lipids, is not understood. Cellular bioenergetics can be influenced by both diabetes and hyperlipidemia and play a pivotal role in the pathophysiology of adult cardiovascular disease. This study investigated whether a maternal high-fat diet, independently or additively with diabetes, could impair fuel metabolism, mitochondrial function, and cardiac physiology in the developing offspring's heart. Sprague-Dawley rats fed a control or high-fat diet were administered placebo or streptozotocin to induce diabetes during pregnancy and then delivered offspring from four groups: control, diabetes exposed, diet exposed, and combination exposed. Cardiac function, cellular bioenergetics (mitochondrial stress test, glycolytic stress test, and palmitate oxidation assay), lipid peroxidation, mitochondrial histology, and copy number were determined. Diabetes-exposed offspring had impaired glycolytic and respiratory capacity and a reduced proton leak. High-fat diet-exposed offspring had increased mitochondrial copy number, increased lipid peroxidation, and evidence of mitochondrial dysfunction. Combination-exposed pups were most severely affected and demonstrated cardiac lipid droplet accumulation and diastolic/systolic cardiac dysfunction that mimics that of adult diabetic cardiomyopathy. This study is the first to demonstrate that a maternal high-fat diet impairs cardiac function in offspring of diabetic pregnancies through metabolic stress and serves as a critical step in understanding the role of cellular bioenergetics in developmentally programmed cardiac disease.
Keywords: cardiac bioenergetics; developmental programming; diabetic cardiomyopathy; diabetic pregnancy; maternal high-fat diet; mitochondrial dysfunction; proton production rate.
Copyright © 2016 the American Physiological Society.
Figures
References
-
- Aman J, Hansson U, Ostlund I, Wall K, Persson B. Increased fat mass and cardiac septal hypertrophy in newborn infants of mothers with well-controlled diabetes during pregnancy. Neonatology 100: 147–154, 2011. - PubMed
-
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 30, September 2001 (replaces Technical Bulletin Number 200, December 1994). Gestational diabetes. Obstet Gynecol 98: 525–538, 2001. - PubMed
-
- Balsells M, Garcia-Patterson A, Gich I, Corcoy R. Maternal and fetal outcome in women with type 2 versus type 1 diabetes mellitus: a systematic review and metaanalysis. J Clin Endocrinol Metab 94: 4284–4291, 2009. - PubMed
-
- Barker DJ. Rise and fall of Western diseases. Nature 338: 371–372, 1989. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

