Bombesin-like receptor 3 regulates blood pressure and heart rate via a central sympathetic mechanism
- PMID: 26801314
- PMCID: PMC4865066
- DOI: 10.1152/ajpheart.00963.2015
Bombesin-like receptor 3 regulates blood pressure and heart rate via a central sympathetic mechanism
Abstract
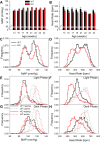
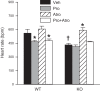
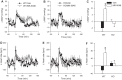
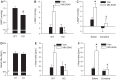
Bombesin-like receptor 3 (BRS-3) is an orphan G protein-coupled receptor that regulates energy expenditure, food intake, and body weight. We examined the effects of BRS-3 deletion and activation on blood pressure and heart rate. In free-living, telemetered Brs3 null mice the resting heart rate was 10% lower than wild-type controls, while the resting mean arterial pressure was unchanged. During physical activity, the heart rate and blood pressure increased more in Brs3 null mice, reaching a similar heart rate and higher mean arterial pressure than control mice. When sympathetic input was blocked with propranolol, the heart rate of Brs3 null mice was unchanged, while the heart rate in control mice was reduced to the level of the null mice. The intrinsic heart rate, measured after both sympathetic and parasympathetic blockade, was similar in Brs3 null and control mice. Intravenous infusion of the BRS-3 agonist MK-5046 increased mean arterial pressure and heart rate in wild-type but not in Brs3 null mice, and this increase was blocked by pretreatment with clonidine, a sympatholytic, centrally acting α2-adrenergic agonist. In anesthetized mice, hypothalamic infusion of MK-5046 also increased both mean arterial pressure and heart rate. Taken together, these data demonstrate that BRS-3 contributes to resting cardiac sympathetic tone, but is not required for activity-induced increases in heart rate and blood pressure. The data suggest that BRS-3 activation increases heart rate and blood pressure via a central sympathetic mechanism.
Keywords: blood pressure; bombesin-like receptor 3; energy metabolism; heart rate; sympathetic nervous system.
Figures
Similar articles
-
Bombesin-Like Receptor 3: Physiology of a Functional Orphan.Trends Endocrinol Metab. 2016 Sep;27(9):603-605. doi: 10.1016/j.tem.2016.03.003. Epub 2016 Apr 4. Trends Endocrinol Metab. 2016. PMID: 27055378 Free PMC article. Review.
-
Regulation of body temperature and brown adipose tissue thermogenesis by bombesin receptor subtype-3.Am J Physiol Endocrinol Metab. 2014 Mar;306(6):E681-7. doi: 10.1152/ajpendo.00615.2013. Epub 2014 Jan 22. Am J Physiol Endocrinol Metab. 2014. PMID: 24452453 Free PMC article.
-
Bombesin-like receptor 3 (Brs3) expression in glutamatergic, but not GABAergic, neurons is required for regulation of energy metabolism.Mol Metab. 2017 Nov;6(11):1540-1550. doi: 10.1016/j.molmet.2017.08.013. Epub 2017 Sep 15. Mol Metab. 2017. PMID: 29107299 Free PMC article.
-
BRS3 in both MC4R- and SIM1-expressing neurons regulates energy homeostasis in mice.Mol Metab. 2020 Jun;36:100969. doi: 10.1016/j.molmet.2020.02.012. Epub 2020 Feb 29. Mol Metab. 2020. PMID: 32229422 Free PMC article.
-
Biology and pharmacology of bombesin receptor subtype-3.Curr Opin Endocrinol Diabetes Obes. 2012 Feb;19(1):3-7. doi: 10.1097/MED.0b013e32834ec77d. Curr Opin Endocrinol Diabetes Obes. 2012. PMID: 22157398 Review.
Cited by
-
Cre Recombinase Driver Mice Reveal Lineage-Dependent and -Independent Expression of Brs3 in the Mouse Brain.eNeuro. 2021 Aug 17;8(4):ENEURO.0252-21.2021. doi: 10.1523/ENEURO.0252-21.2021. Print 2021 Jul-Aug. eNeuro. 2021. PMID: 34326065 Free PMC article.
-
Bombesin receptor subtype-3-expressing neurons regulate energy homeostasis through a novel neuronal pathway in the hypothalamus.Brain Behav. 2017 Dec 15;8(1):e00881. doi: 10.1002/brb3.881. eCollection 2018 Jan. Brain Behav. 2017. PMID: 29568682 Free PMC article.
-
Brs3 neurons in the mouse dorsomedial hypothalamus regulate body temperature, energy expenditure, and heart rate, but not food intake.Nat Neurosci. 2018 Nov;21(11):1530-1540. doi: 10.1038/s41593-018-0249-3. Epub 2018 Oct 22. Nat Neurosci. 2018. PMID: 30349101 Free PMC article.
-
Gqα/G11α deficiency in dorsomedial hypothalamus leads to obesity resulting from decreased energy expenditure and impaired sympathetic nerve activity.Am J Physiol Endocrinol Metab. 2021 Feb 1;320(2):E270-E280. doi: 10.1152/ajpendo.00059.2020. Epub 2020 Nov 9. Am J Physiol Endocrinol Metab. 2021. PMID: 33166186 Free PMC article.
-
Bombesin-Like Receptor 3: Physiology of a Functional Orphan.Trends Endocrinol Metab. 2016 Sep;27(9):603-605. doi: 10.1016/j.tem.2016.03.003. Epub 2016 Apr 4. Trends Endocrinol Metab. 2016. PMID: 27055378 Free PMC article. Review.
References
-
- Blair ML, Mickelsen D. Activation of lateral parabrachial nucleus neurons restores blood pressure and sympathetic vasomotor drive after hypotensive hemorrhage. Am J Physiol Regul Integr Comp Physiol 291: R742–R750, 2006. - PubMed
-
- Culman J, Unger T. Central mechanisms regulating blood pressure: circuits and transmitters. Eur Heart J 13, Suppl A: 10–17, 1992. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

