γ-Tocopherol supplementation of allergic female mice augments development of CD11c+CD11b+ dendritic cells in utero and allergic inflammation in neonates
- PMID: 26801566
- PMCID: PMC4836111
- DOI: 10.1152/ajplung.00301.2015
γ-Tocopherol supplementation of allergic female mice augments development of CD11c+CD11b+ dendritic cells in utero and allergic inflammation in neonates
Abstract
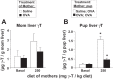
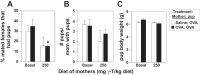
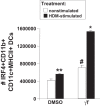
γ-Tocopherol increases responses to allergen challenge in allergic adult mice, but it is not known whether γ-tocopherol regulates the development of allergic disease. Development of allergic disease often occurs early in life. In clinical studies and animal models, offspring of allergic mothers have increased responsiveness to allergen challenge. Therefore, we determined whether γ-tocopherol augments development of allergic responses in offspring of allergic female mice. Allergic female mice were supplemented with γ-tocopherol starting at mating. The pups from allergic mothers developed allergic lung responses, whereas pups from saline-treated mothers did not respond to allergen challenge. The γ-tocopherol supplementation of allergic female mice increased the numbers of eosinophils twofold in the pup bronchoalveolar lavage and lungs after allergen challenge. There was also about a twofold increase in pup lung CD11b(+) subsets of CD11c(+) dendritic cells and in numbers of these dendritic cells expressing the transcription factor IRF4. There was no change in several CD11b(-) dendritic cell subsets. Furthermore, maternal supplementation with γ-tocopherol increased the number of fetal liver CD11b(+)CD11c(+) dendritic cells twofold in utero. In the pups, γ-tocopherol increased lung expression of the inflammatory mediators CCL11, amphiregulin, activin A, and IL-5. In conclusion, maternal supplementation with γ-tocopherol increased fetal development of subsets of dendritic cells that are critical for allergic responses and increased development of allergic responses in pups from allergic mothers. These results have implications for supplementation of allergic mothers with γ-tocopherol in prenatal vitamins.
Keywords: allergic lung inflammation; dendritic cells; vitamin E; γ-tocopherol.
Copyright © 2016 the American Physiological Society.
Figures







Similar articles
-
α-Tocopherol supplementation of allergic female mice inhibits development of CD11c+CD11b+ dendritic cells in utero and allergic inflammation in neonates.Am J Physiol Lung Cell Mol Physiol. 2014 Sep 15;307(6):L482-96. doi: 10.1152/ajplung.00132.2014. Epub 2014 Jul 11. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 25015974 Free PMC article.
-
β-Glucosylceramides and Tocopherols Regulate Development and Function of Dendritic Cells.J Immunol. 2022 Nov 15;209(10):1837-1850. doi: 10.4049/jimmunol.2101188. J Immunol. 2022. PMID: 36426950 Free PMC article.
-
Dendritic cell-specific deletion of PKCδ in offspring of allergic mothers prevents the predisposition for development of allergic lung inflammation in offspring.J Leukoc Biol. 2024 Nov 27;116(6):1432-1445. doi: 10.1093/jleuko/qiae207. J Leukoc Biol. 2024. PMID: 39312649
-
Vitamin E isoforms as modulators of lung inflammation.Nutrients. 2013 Oct 31;5(11):4347-63. doi: 10.3390/nu5114347. Nutrients. 2013. PMID: 24184873 Free PMC article. Review.
-
Maternal allergen exposure as a risk factor for childhood asthma.Curr Allergy Asthma Rep. 2006 Feb;6(1):75-80. doi: 10.1007/s11882-006-0014-7. Curr Allergy Asthma Rep. 2006. PMID: 16476199 Review.
Cited by
-
Associations of α- and γ-tocopherol during early life with lung function in childhood.J Allergy Clin Immunol. 2020 Dec;146(6):1349-1357.e3. doi: 10.1016/j.jaci.2020.04.019. Epub 2020 Apr 25. J Allergy Clin Immunol. 2020. PMID: 32344059 Free PMC article. Clinical Trial.
-
Delineation of the Individual Effects of Vitamin E Isoforms on Early Life Incident Wheezing.J Pediatr. 2019 Mar;206:156-163.e3. doi: 10.1016/j.jpeds.2018.10.045. Epub 2018 Dec 5. J Pediatr. 2019. PMID: 30527752 Free PMC article.
-
Mechanism for initiation of food allergy: Dependence on skin barrier mutations and environmental allergen costimulation.J Allergy Clin Immunol. 2018 May;141(5):1711-1725.e9. doi: 10.1016/j.jaci.2018.02.003. Epub 2018 Feb 15. J Allergy Clin Immunol. 2018. PMID: 29454836 Free PMC article.
-
Oxidative Stress: Harms and Benefits for Human Health.Oxid Med Cell Longev. 2017;2017:8416763. doi: 10.1155/2017/8416763. Epub 2017 Jul 27. Oxid Med Cell Longev. 2017. PMID: 28819546 Free PMC article. Review.
-
The Role of Vitamin E in Immunity.Nutrients. 2018 Nov 1;10(11):1614. doi: 10.3390/nu10111614. Nutrients. 2018. PMID: 30388871 Free PMC article. Review.
References
-
- Abdala-Valencia H, Earwood J, Bansal S, Jansen M, Babcock G, Garvy B, Wills-Karp M, Cook-Mills JM. Nonhematopoietic NADPH oxidase regulation of lung eosinophilia and airway hyperresponsiveness in experimentally induced asthma. Am J Physiol Lung Cell Mol Physiol 292: L1111–L1125, 2007. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

