Electrolyte transport properties in distal small airways from cystic fibrosis pigs with implications for host defense
- PMID: 26801568
- PMCID: PMC4824164
- DOI: 10.1152/ajplung.00422.2015
Electrolyte transport properties in distal small airways from cystic fibrosis pigs with implications for host defense
Abstract
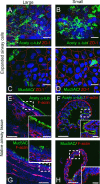
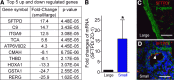
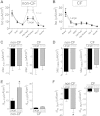
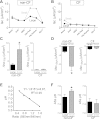
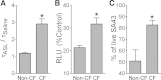
While pathological and clinical data suggest that small airways are involved in early cystic fibrosis (CF) lung disease development, little is known about how the lack of cystic fibrosis transmembrane conductance regulator (CFTR) function contributes to disease pathogenesis in these small airways. Large and small airway epithelia are exposed to different airflow velocities, temperatures, humidity, and CO2 concentrations. The cellular composition of these two regions is different, and small airways lack submucosal glands. To better understand the ion transport properties and impacts of lack of CFTR function on host defense function in small airways, we adapted a novel protocol to isolate small airway epithelial cells from CF and non-CF pigs and established an organotypic culture model. Compared with non-CF large airways, non-CF small airway epithelia cultures had higher Cl(-) and bicarbonate (HCO3 (-)) short-circuit currents and higher airway surface liquid (ASL) pH under 5% CO2 conditions. CF small airway epithelia were characterized by minimal Cl(-) and HCO3 (-) transport and decreased ASL pH, and had impaired bacterial killing compared with non-CF small airways. In addition, CF small airway epithelia had a higher ASL viscosity than non-CF small airways. Thus, the activity of CFTR is higher in the small airways, where it plays a role in alkalinization of ASL, enhancement of antimicrobial activity, and lowering of mucus viscosity. These data provide insight to explain why the small airways are a susceptible site for the bacterial colonization.
Keywords: cystic fibrosis transmembrane conductance regulator.
Copyright © 2016 the American Physiological Society.
Figures

Similar articles
-
Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung.Nature. 2012 Jul 4;487(7405):109-13. doi: 10.1038/nature11130. Nature. 2012. PMID: 22763554 Free PMC article.
-
Acidic pH increases airway surface liquid viscosity in cystic fibrosis.J Clin Invest. 2016 Mar 1;126(3):879-91. doi: 10.1172/JCI83922. Epub 2016 Jan 25. J Clin Invest. 2016. PMID: 26808501 Free PMC article.
-
Inflammatory cytokines TNF-α and IL-17 enhance the efficacy of cystic fibrosis transmembrane conductance regulator modulators.J Clin Invest. 2021 Aug 16;131(16):e150398. doi: 10.1172/JCI150398. J Clin Invest. 2021. PMID: 34166230 Free PMC article.
-
HCO3- transport in relation to mucus secretion from submucosal glands.JOP. 2001 Jul;2(4 Suppl):280-4. JOP. 2001. PMID: 11875272 Review.
-
Pharmacotherapy of the ion transport defect in cystic fibrosis: role of purinergic receptor agonists and other potential therapeutics.Am J Respir Med. 2003;2(4):299-309. doi: 10.1007/BF03256658. Am J Respir Med. 2003. PMID: 14719996 Review.
Cited by
-
Protein Kinase Cζ Inhibitor Promotes Resolution of Bleomycin-Induced Acute Lung Injury.Am J Respir Cell Mol Biol. 2016 Dec;55(6):869-877. doi: 10.1165/rcmb.2015-0006OC. Am J Respir Cell Mol Biol. 2016. PMID: 27486964 Free PMC article.
-
Enhanced Tropism of Species B1 Adenoviral-Based Vectors for Primary Human Airway Epithelial Cells.Mol Ther Methods Clin Dev. 2019 Jul 12;14:228-236. doi: 10.1016/j.omtm.2019.07.001. eCollection 2019 Sep 13. Mol Ther Methods Clin Dev. 2019. PMID: 31417941 Free PMC article.
-
V-Type ATPase Mediates Airway Surface Liquid Acidification in Pig Small Airway Epithelial Cells.Am J Respir Cell Mol Biol. 2021 Aug;65(2):146-156. doi: 10.1165/rcmb.2020-0349OC. Am J Respir Cell Mol Biol. 2021. PMID: 33789071 Free PMC article.
-
CFTR Protein: Not Just a Chloride Channel?Cells. 2021 Oct 22;10(11):2844. doi: 10.3390/cells10112844. Cells. 2021. PMID: 34831067 Free PMC article. Review.
-
Transduction of Pig Small Airway Epithelial Cells and Distal Lung Progenitor Cells by AAV4.Cells. 2021 Apr 25;10(5):1014. doi: 10.3390/cells10051014. Cells. 2021. PMID: 33923029 Free PMC article.
References
-
- Adam RJ, Michalski AS, Bauer C, Abou Alaiwa MH, Gross TJ, Awadalla MS, Bouzek DC, Gansemer ND, Taft PJ, Hoegger MJ, Diwakar A, Ochs M, Reinhardt JM, Hoffman EA, Beichel RR, Meyerholz DK, Stoltz DA. Air trapping and airflow obstruction in newborn cystic fibrosis piglets. Am J Respir Crit Care Med 188: 1434–1441, 2013. - PMC - PubMed
-
- Al-Bazzaz FJ, Hafez N, Tyagi S, Gailey CA, Toofanfard M, Alrefai WA, Nazir TM, Ramaswamy K, Dudeja PK. Detection of Cl–HCO3− and Na+-H+ exchangers in human airways epithelium. JOP 2: 285–290, 2001. - PubMed
-
- Asmundsson T, Kilburn KH. Mucociliary clearance rates at various levels in dog lungs. Am Rev Respir Dis 102: 388–397, 1970. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

