Molecular Structural Basis for the Cold Adaptedness of the Psychrophilic β-Glucosidase BglU in Micrococcus antarcticus
- PMID: 26801571
- PMCID: PMC4807509
- DOI: 10.1128/AEM.03158-15
Molecular Structural Basis for the Cold Adaptedness of the Psychrophilic β-Glucosidase BglU in Micrococcus antarcticus
Abstract
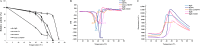
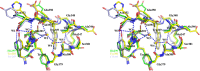
Psychrophilic enzymes play crucial roles in cold adaptation of microbes and provide useful models for studies of protein evolution, folding, and dynamic properties. We examined the crystal structure (2.2-Å resolution) of the psychrophilic β-glucosidase BglU, a member of the glycosyl hydrolase 1 (GH1) enzyme family found in the cold-adapted bacterium Micrococcus antarcticus. Structural comparison and sequence alignment between BglU and its mesophilic and thermophilic counterpart enzymes (BglB and GlyTn, respectively) revealed two notable features distinct to BglU: (i) a unique long-loop L3 (35 versus 7 amino acids in others) involved in substrate binding and (ii) a unique amino acid, His299 (Tyr in others), involved in the stabilization of an ordered water molecule chain. Shortening of loop L3 to 25 amino acids reduced low-temperature catalytic activity, substrate-binding ability, the optimal temperature, and the melting temperature (Tm). Mutation of His299 to Tyr increased the optimal temperature, the Tm, and the catalytic activity. Conversely, mutation of Tyr301 to His in BglB caused a reduction in catalytic activity, thermostability, and the optimal temperature (45 to 35°C). Loop L3 shortening and H299Y substitution jointly restored enzyme activity to the level of BglU, but at moderate temperatures. Our findings indicate that loop L3 controls the level of catalytic activity at low temperatures, residue His299 is responsible for thermolability (particularly heat lability of the active center), and long-loop L3 and His299 are jointly responsible for the psychrophilic properties. The described structural basis for the cold adaptedness of BglU will be helpful for structure-based engineering of new cold-adapted enzymes and for the production of mutants useful in a variety of industrial processes at different temperatures.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
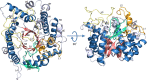



Similar articles
-
Specific amino acids responsible for the cold adaptedness of Micrococcus antarcticus β-glucosidase BglU.Appl Microbiol Biotechnol. 2017 Mar;101(5):2033-2041. doi: 10.1007/s00253-016-7990-x. Epub 2016 Nov 17. Appl Microbiol Biotechnol. 2017. PMID: 27858137
-
Gene cloning and characterization of a cold-adapted β-glucosidase belonging to glycosyl hydrolase family 1 from a psychrotolerant bacterium Micrococcus antarcticus.Enzyme Microb Technol. 2011 Jun 10;49(1):94-9. doi: 10.1016/j.enzmictec.2011.03.001. Epub 2011 Mar 21. Enzyme Microb Technol. 2011. PMID: 22112277
-
A novel low-temperature-active β-glucosidase from symbiotic Serratia sp. TN49 reveals four essential positions for substrate accommodation.Appl Microbiol Biotechnol. 2011 Oct;92(2):305-15. doi: 10.1007/s00253-011-3323-2. Epub 2011 May 11. Appl Microbiol Biotechnol. 2011. PMID: 21559826
-
Molecular adaptations to cold in psychrophilic enzymes.Cell Mol Life Sci. 2003 Apr;60(4):648-62. doi: 10.1007/s00018-003-2155-3. Cell Mol Life Sci. 2003. PMID: 12785714 Free PMC article. Review.
-
Psychrophilic enzymes: molecular basis of cold adaptation.Cell Mol Life Sci. 1997 Oct;53(10):830-41. doi: 10.1007/s000180050103. Cell Mol Life Sci. 1997. PMID: 9413552 Free PMC article. Review.
Cited by
-
The Conformational Change of the L3 Loop Affects the Structural Changes in the Substrate Binding Pocket Entrance of β-Glucosidase.Molecules. 2023 Nov 27;28(23):7807. doi: 10.3390/molecules28237807. Molecules. 2023. PMID: 38067537 Free PMC article.
-
Improving the Specific Activity and Thermostability of Psychrophilic Xylosidase AX543 by Comparative Mutagenesis.Foods. 2022 Aug 16;11(16):2463. doi: 10.3390/foods11162463. Foods. 2022. PMID: 36010463 Free PMC article.
-
Recent Development of Extremophilic Bacteria and Their Application in Biorefinery.Front Bioeng Biotechnol. 2020 Jun 12;8:483. doi: 10.3389/fbioe.2020.00483. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32596215 Free PMC article. Review.
-
Establishment of mesophilic-like catalytic properties in a thermophilic enzyme without affecting its thermal stability.Sci Rep. 2019 Jun 27;9(1):9346. doi: 10.1038/s41598-019-45560-x. Sci Rep. 2019. PMID: 31249343 Free PMC article.
-
Biochemical and Structural Analysis of a Glucose-Tolerant β-Glucosidase from the Hemicellulose-Degrading Thermoanaerobacterium saccharolyticum.Molecules. 2022 Jan 4;27(1):290. doi: 10.3390/molecules27010290. Molecules. 2022. PMID: 35011521 Free PMC article.
References
-
- Chuenchor W, Pengthaisong S, Robinson RC, Yuvaniyama J, Oonanant W, Bevan DR, Esen A, Chen CJ, Opassiri R, Svasti J, Cairns JR. 2008. Structural insights into rice BGlu1 β-glucosidase oligosaccharide hydrolysis and transglycosylation. J Mol Biol 377:1200–1215. doi:10.1016/j.jmb.2008.01.076. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

