The Arabidopsis AtPP2CA Protein Phosphatase Inhibits the GORK K+ Efflux Channel and Exerts a Dominant Suppressive Effect on Phosphomimetic-activating Mutations
- PMID: 26801610
- PMCID: PMC4813591
- DOI: 10.1074/jbc.M115.711309
The Arabidopsis AtPP2CA Protein Phosphatase Inhibits the GORK K+ Efflux Channel and Exerts a Dominant Suppressive Effect on Phosphomimetic-activating Mutations
Abstract
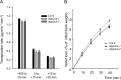
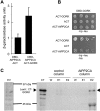
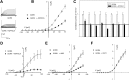
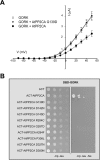
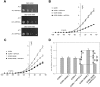
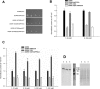
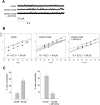
The regulation of the GORK (Guard Cell Outward Rectifying) Shaker channel mediating a massive K(+) efflux in Arabidopsis guard cells by the phosphatase AtPP2CA was investigated. Unlike the gork mutant, the atpp2ca mutants displayed a phenotype of reduced transpiration. We found that AtPP2CA interacts physically with GORK and inhibits GORK activity in Xenopus oocytes. Several amino acid substitutions in the AtPP2CA active site, including the dominant interfering G145D mutation, disrupted the GORK-AtPP2CA interaction, meaning that the native conformation of the AtPP2CA active site is required for the GORK-AtPP2CA interaction. Furthermore, two serines in the GORK ankyrin domain that mimic phosphorylation (Ser to Glu) or dephosphorylation (Ser to Ala) were mutated. Mutations mimicking phosphorylation led to a significant increase in GORK activity, whereas mutations mimicking dephosphorylation had no effect on GORK. In Xenopus oocytes, the interaction of AtPP2CA with "phosphorylated" or "dephosphorylated" GORK systematically led to inhibition of the channel to the same baseline level. Single-channel recordings indicated that the GORK S722E mutation increases the open probability of the channel in the absence, but not in the presence, of AtPP2CA. The dephosphorylation-independent inactivation mechanism of GORK by AtPP2CA is discussed in relation with well known conformational changes in animal Shaker-like channels that lead to channel opening and closing. In plants, PP2C activity would control the stomatal aperture by regulating both GORK and SLAC1, the two main channels required for stomatal closure.
Keywords: Arabidopsis thaliana; Xenopus; abscisic acid (ABA); mutagenesis; phosphatase; plant molecular biology; plant physiology; potassium channel; potassium transport; protein-protein interaction.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

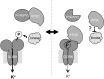
Similar articles
-
Physical and functional interaction of the Arabidopsis K(+) channel AKT2 and phosphatase AtPP2CA.Plant Cell. 2002 May;14(5):1133-46. doi: 10.1105/tpc.000943. Plant Cell. 2002. PMID: 12034902 Free PMC article.
-
The protein phosphatase AtPP2CA negatively regulates abscisic acid signal transduction in Arabidopsis, and effects of abh1 on AtPP2CA mRNA.Plant Physiol. 2006 Jan;140(1):127-39. doi: 10.1104/pp.105.070318. Epub 2005 Dec 16. Plant Physiol. 2006. PMID: 16361522 Free PMC article.
-
Functional identification of a GORK potassium channel from the ancient desert shrub Ammopiptanthus mongolicus (Maxim.) Cheng f.Plant Cell Rep. 2016 Apr;35(4):803-15. doi: 10.1007/s00299-015-1922-6. Epub 2016 Jan 25. Plant Cell Rep. 2016. PMID: 26804987
-
Signal transduction and ion channels in guard cells.Philos Trans R Soc Lond B Biol Sci. 1998 Sep 29;353(1374):1475-88. doi: 10.1098/rstb.1998.0303. Philos Trans R Soc Lond B Biol Sci. 1998. PMID: 9800209 Free PMC article. Review.
-
The guard cell as a single-cell model towards understanding drought tolerance and abscisic acid action.J Exp Bot. 2009;60(5):1439-63. doi: 10.1093/jxb/ern340. Epub 2009 Jan 30. J Exp Bot. 2009. PMID: 19181866 Review.
Cited by
-
Morphophysiological and transcriptome analysis reveal that reprogramming of metabolism, phytohormones and root development pathways governs the potassium (K+) deficiency response in two contrasting chickpea cultivars.Front Plant Sci. 2023 Jan 11;13:1054821. doi: 10.3389/fpls.2022.1054821. eCollection 2022. Front Plant Sci. 2023. PMID: 36714783 Free PMC article.
-
Structural and Functional Insights into the Role of Guard Cell Ion Channels in Abiotic Stress-Induced Stomatal Closure.Plants (Basel). 2021 Dec 15;10(12):2774. doi: 10.3390/plants10122774. Plants (Basel). 2021. PMID: 34961246 Free PMC article. Review.
-
Recognition and Activation of the Plant AKT1 Potassium Channel by the Kinase CIPK23.Plant Physiol. 2020 Apr;182(4):2143-2153. doi: 10.1104/pp.19.01084. Epub 2020 Feb 3. Plant Physiol. 2020. PMID: 32015077 Free PMC article.
-
The Membrane Transport System of the Guard Cell and Its Integration for Stomatal Dynamics.Plant Physiol. 2017 Jun;174(2):487-519. doi: 10.1104/pp.16.01949. Epub 2017 Apr 13. Plant Physiol. 2017. PMID: 28408539 Free PMC article. Review.
-
Adjustment of K+ Fluxes and Grapevine Defense in the Face of Climate Change.Int J Mol Sci. 2021 Sep 27;22(19):10398. doi: 10.3390/ijms221910398. Int J Mol Sci. 2021. PMID: 34638737 Free PMC article. Review.
References
-
- Leung J., Bouvier-Durand M., Morris P. C., Guerrier D., Chefdor F., and Giraudat J. (1994) Arabidopsis ABA response gene ABI1: features of a calcium-modulated protein phosphatase. Science 264, 1448–1452 - PubMed
-
- Meyer K., Leube M. P., and Grill E. (1994) A protein phosphatase 2C involved in ABA signal transduction in Arabidopsis thaliana. Science 264, 1452–1455 - PubMed
-
- Schweighofer A., Hirt H., and Meskiene I. (2004) Plant PP2C phosphatases: emerging functions in stress signaling. Trends Plant Sci. 9, 236–243 - PubMed
-
- Merlot S., Gosti F., Guerrier D., Vavasseur A., and Giraudat J. (2001) The ABI1 and ABI2 protein phosphatases 2C act in a negative feedback regulatory loop of the abscisic acid signalling pathway. Plant J. 25, 295–303 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

