The Contribution of Non-catalytic Carbohydrate Binding Modules to the Activity of Lytic Polysaccharide Monooxygenases
- PMID: 26801613
- PMCID: PMC4817175
- DOI: 10.1074/jbc.M115.702365
The Contribution of Non-catalytic Carbohydrate Binding Modules to the Activity of Lytic Polysaccharide Monooxygenases
Abstract
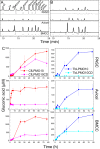
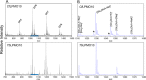
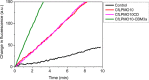
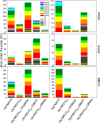
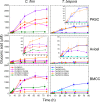
Lignocellulosic biomass is a sustainable industrial substrate. Copper-dependent lytic polysaccharide monooxygenases (LPMOs) contribute to the degradation of lignocellulose and increase the efficiency of biofuel production. LPMOs can contain non-catalytic carbohydrate binding modules (CBMs), but their role in the activity of these enzymes is poorly understood. Here we explored the importance of CBMs in LPMO function. The family 2a CBMs of two monooxygenases,CfLPMO10 andTbLPMO10 fromCellulomonas fimiandThermobispora bispora, respectively, were deleted and/or replaced with CBMs from other proteins. The data showed that the CBMs could potentiate and, surprisingly, inhibit LPMO activity, and that these effects were both enzyme-specific and substrate-specific. Removing the natural CBM or introducingCtCBM3a, from theClostridium thermocellumcellulosome scaffoldin CipA, almost abolished the catalytic activity of the LPMOs against the cellulosic substrates. The deleterious effect of CBM removal likely reflects the importance of prolonged presentation of the enzyme on the surface of the substrate for efficient catalytic activity, as only LPMOs appended to CBMs bound tightly to cellulose. The negative impact ofCtCBM3a is in sharp contrast with the capacity of this binding module to potentiate the activity of a range of glycoside hydrolases including cellulases. The deletion of the endogenous CBM fromCfLPMO10 or the introduction of a family 10 CBM fromCellvibrio japonicusLPMO10B intoTbLPMO10 influenced the quantity of non-oxidized products generated, demonstrating that CBMs can modulate the mode of action of LPMOs. This study demonstrates that engineered LPMO-CBM hybrids can display enhanced industrially relevant oxygenations.
Keywords: carbohydrate binding modules; carbohydrate-binding protein; cellulases; enzyme; glycoside hydrolase; lignocellulose degradation; lytic polysaccharide monooxygenases; plant cell wall; protein engineering.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Himmel M. E., Ding S. Y., Johnson D. K., Adney W. S., Nimlos M. R., Brady J. W., and Foust T. D. (2007) Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science 315, 804–807 - PubMed
-
- Quinlan R. J., Sweeney M. D., Lo Leggio L., Otten H., Poulsen J. C., Johansen K. S., Krogh K. B., Jørgensen C. I., Tovborg M., Anthonsen A., Tryfona T., Walter C. P., Dupree P., Xu F., Davies G. J., and Walton P. H. (2011) Insights into the oxidative degradation of cellulose by a copper metalloenzyme that exploits biomass components. Proc. Natl. Acad. Sci. U.S.A. 108, 15079–15084 - PMC - PubMed
-
- Harris P. V., Welner D., McFarland K. C., Re E., Navarro Poulsen J. C., Brown K., Salbo R., Ding H., Vlasenko E., Merino S., Xu F., Cherry J., Larsen S., and Lo Leggio L. (2010) Stimulation of lignocellulosic biomass hydrolysis by proteins of glycoside hydrolase family 61: structure and function of a large, enigmatic family. Biochemistry 49, 3305–3316 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

