A Simultaneous Genetic Screen for Zygotic and Sterile Mutants in a Hermaphroditic Vertebrate (Kryptolebias marmoratus)
- PMID: 26801648
- PMCID: PMC4825645
- DOI: 10.1534/g3.115.022475
A Simultaneous Genetic Screen for Zygotic and Sterile Mutants in a Hermaphroditic Vertebrate (Kryptolebias marmoratus)
Abstract
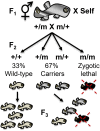
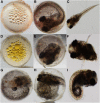
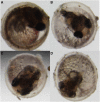
The mangrove killifish, Kryptolebias marmoratus, is unique among vertebrates due to its self-fertilizing mode of reproduction involving an ovotestis. As a result, it constitutes a simplistic and desirable vertebrate model for developmental genetics as it is easily maintained, reaches sexual maturity in about 100 days, and provides a manageable number of relatively clear embryos. After the establishment and characterization of an initial mutagenesis pilot screen using N-ethyl-N-nitrosourea, a three-generation genetic screen was performed to confirm zygotic mutant allele heritability and simultaneously score for homozygous recessive mutant sterile F2 fish. From a total of 307 F2 fish screened, 10 were found to be 1° males, 16 were sterile, 92 wild-type, and the remaining 189, carriers of zygotic recessive alleles. These carriers produced 25% progeny exhibiting several zygotic phenotypes similar to those previously described in zebrafish and in the aforementioned pilot screen, as expected. Interestingly, new phenotypes such as golden yolk, no trunk, and short tail were observed. The siblings of sterile F2 mutants were used to produce an F3 generation in order to confirm familial sterility. Out of the 284 F3 fish belonging to 10 previously identified sterile families, 12 were found to be 1° males, 69 were wild-type, 83 sterile, and 120 were classified as */+ (either wild-type or carriers) with undefined genotypes. This screen provides proof of principle that K. marmoratus is a powerful vertebrate model for developmental genetics and can be used to identify mutations affecting fertility.
Keywords: ENU mutagenesis; Kryptolebias marmoratus; forward genetic screen; sterile mutant; zygotic mutant.
Copyright © 2016 Sucar et al.
Figures






Similar articles
-
Establishing developmental genetics in a self-fertilizing fish (Krytolebias marmoratus).Integr Comp Biol. 2012 Dec;52(6):781-91. doi: 10.1093/icb/ics052. Epub 2012 Apr 27. Integr Comp Biol. 2012. PMID: 22544288 Free PMC article.
-
Population genetics and evolution of the mangrove rivulus Kryptolebias marmoratus, the world's only self-fertilizing hermaphroditic vertebrate.J Fish Biol. 2015 Sep;87(3):519-38. doi: 10.1111/jfb.12741. Epub 2015 Jul 29. J Fish Biol. 2015. PMID: 26223378 Review.
-
Embryonic development of the self-fertilizing mangrove killifish Kryptolebias marmoratus.Dev Dyn. 2011 Jul;240(7):1694-704. doi: 10.1002/dvdy.22668. Dev Dyn. 2011. PMID: 21674684
-
Genetic tools for the study of the mangrove killifish, Kryptolebias marmoratus, an emerging vertebrate model for phenotypic plasticity.J Exp Zool B Mol Dev Evol. 2024 May;342(3):164-177. doi: 10.1002/jez.b.23216. Epub 2023 Aug 8. J Exp Zool B Mol Dev Evol. 2024. PMID: 37553824
-
Whole genome data for omics-based research on the self-fertilizing fish Kryptolebias marmoratus.Mar Pollut Bull. 2014 Aug 30;85(2):532-41. doi: 10.1016/j.marpolbul.2014.04.005. Epub 2014 Apr 20. Mar Pollut Bull. 2014. PMID: 24759509 Review.
Cited by
-
Developmental defects in ectodermal appendages caused by missense mutation in edaradd gene in the nfr mangrove killifish kryptolebias marmoratus.Sci Rep. 2025 Jan 21;15(1):2660. doi: 10.1038/s41598-024-82276-z. Sci Rep. 2025. PMID: 39837941 Free PMC article.
-
A Genetic Map for the Only Self-Fertilizing Vertebrate.G3 (Bethesda). 2016 Apr 7;6(4):1095-106. doi: 10.1534/g3.115.022699. G3 (Bethesda). 2016. PMID: 26865699 Free PMC article.
-
Molecular mechanisms of embryonic tail development in the self-fertilizing mangrove killifish Kryptolebias marmoratus.Development. 2021 Dec 15;148(24):dev199675. doi: 10.1242/dev.199675. Epub 2021 Dec 24. Development. 2021. PMID: 34951463 Free PMC article.
-
The Genome of the Self-Fertilizing Mangrove Rivulus Fish, Kryptolebias marmoratus: A Model for Studying Phenotypic Plasticity and Adaptations to Extreme Environments.Genome Biol Evol. 2016 Aug 16;8(7):2145-54. doi: 10.1093/gbe/evw145. Genome Biol Evol. 2016. PMID: 27324916 Free PMC article.
References
-
- Amacher S. L., Draper B. W., Summers C. B., 2002. The zebrafish T-box genes no tail and spadetail are required for development of trunk and tail mesoderm and medial floor plate. Development 129: 3311–3323. - PubMed
-
- Avise J. C., Tatarenkov A., 2015. Population genetics and evolution of the mangrove rivulus Kryptolebias marmoratus the world’s only self-fertilizing hermaphroditic vertebrate. J. Fish Biol. 87(3): 519–538. - PubMed
-
- Bashamboo A., McElreavey K., 2014. Consanguinity and disorders of sex development. Hum. Hered. 77: 108–117. - PubMed
-
- Bauer M. P., Goetz F. W., 2001. Isolation of gonadal mutations in adult zebrafish from a chemical mutagenesis screen. Biol. Reprod. 64: 548–554. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
