Overcoming cisplatin resistance of ovarian cancer cells by targeting HIF-1-regulated cancer metabolism
- PMID: 26801746
- PMCID: PMC4769873
- DOI: 10.1016/j.canlet.2016.01.009
Overcoming cisplatin resistance of ovarian cancer cells by targeting HIF-1-regulated cancer metabolism
Abstract
Cisplatin is currently one of the most effective chemotherapeutic drugs used for treating ovarian cancer; however, resistance to cisplatin is common. In this study, we explored an experimental strategy for overcoming cisplatin resistance of human ovarian cancer from the new perspective of cancer cell metabolism. By using two pairs of genetically matched cisplatin-sensitive and cisplatin-resistant ovarian cancer cell lines, we tested the hypothesis that downregulating hypoxia-inducible factor-1 (HIF-1), which regulates metabolic enzymes involved in glycolysis, is a promising strategy for overcoming cisplatin resistance of human ovarian cancer cells. We found that cisplatin downregulated the level of the regulatable α subunit of HIF-1, HIF-1α, in cisplatin-sensitive ovarian cancer cells through enhancing HIF-1α degradation but did not downregulate HIF-1α in their cisplatin-resistant counterparts. Overexpression of a degradation-resistant HIF-1α (HIF-1α ΔODD) reduced cisplatin-induced apoptosis in cisplatin-sensitive cells, whereas genetic knockdown of HIF-1α or pharmacological promotion of HIF-1α degradation enhanced response to cisplatin in both cisplatin-sensitive and cisplatin-resistant ovarian cancer cells. We further demonstrated that knockdown of HIF-1α improved the response of cisplatin-resistant ovarian cancer cells to cisplatin by redirecting the aerobic glycolysis in the resistant cancer cells toward mitochondrial oxidative phosphorylation, leading to cell death through overproduction of reactive oxygen species. Our findings suggest that the HIF-1α-regulated cancer metabolism pathway could be a novel target for overcoming cisplatin resistance in ovarian cancer.
Keywords: Cancer metabolism; Cisplatin; HIF-1; Ovarian cancer; Resistance.
Copyright © 2016 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
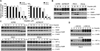
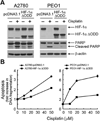



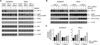
Similar articles
-
ABT737 reverses cisplatin resistance by targeting glucose metabolism of human ovarian cancer cells.Int J Oncol. 2018 Sep;53(3):1055-1068. doi: 10.3892/ijo.2018.4476. Epub 2018 Jul 9. Int J Oncol. 2018. PMID: 30015875 Free PMC article.
-
Knockdown of HIF-1α by siRNA-expressing plasmid delivered by attenuated Salmonella enhances the antitumor effects of cisplatin on prostate cancer.Sci Rep. 2017 Aug 8;7(1):7546. doi: 10.1038/s41598-017-07973-4. Sci Rep. 2017. PMID: 28790395 Free PMC article.
-
Identifying novel hypoxia-associated markers of chemoresistance in ovarian cancer.BMC Cancer. 2015 Jul 25;15:547. doi: 10.1186/s12885-015-1539-8. BMC Cancer. 2015. PMID: 26205780 Free PMC article.
-
HIF-1alpha modulates energy metabolism in cancer cells by inducing over-expression of specific glycolytic isoforms.Mini Rev Med Chem. 2009 Aug;9(9):1084-101. doi: 10.2174/138955709788922610. Mini Rev Med Chem. 2009. PMID: 19689405 Review.
-
Stress-Adaptive Response in Ovarian Cancer Drug Resistance: Role of TRAP1 in Oxidative Metabolism-Driven Inflammation.Adv Protein Chem Struct Biol. 2017;108:163-198. doi: 10.1016/bs.apcsb.2017.01.004. Epub 2017 Feb 12. Adv Protein Chem Struct Biol. 2017. PMID: 28427560 Review.
Cited by
-
ASCT2 (SLC1A5) is an EGFR-associated protein that can be co-targeted by cetuximab to sensitize cancer cells to ROS-induced apoptosis.Cancer Lett. 2016 Oct 10;381(1):23-30. doi: 10.1016/j.canlet.2016.07.020. Epub 2016 Jul 19. Cancer Lett. 2016. PMID: 27450723 Free PMC article.
-
Berberine as a potential agent for breast cancer therapy.Front Oncol. 2022 Sep 2;12:993775. doi: 10.3389/fonc.2022.993775. eCollection 2022. Front Oncol. 2022. PMID: 36119505 Free PMC article. Review.
-
Acetyl-CoA carboxylase rewires cancer metabolism to allow cancer cells to survive inhibition of the Warburg effect by cetuximab.Cancer Lett. 2017 Jan 1;384:39-49. doi: 10.1016/j.canlet.2016.09.020. Epub 2016 Sep 28. Cancer Lett. 2017. PMID: 27693630 Free PMC article.
-
Autophagy as an emerging therapy target for ovarian carcinoma.Oncotarget. 2016 Dec 13;7(50):83476-83487. doi: 10.18632/oncotarget.13080. Oncotarget. 2016. PMID: 27825125 Free PMC article. Review.
-
Hypoxia-induced m6A demethylase ALKBH5 promotes ovarian cancer tumorigenicity by decreasing methylation of the lncRNA RMRP.Am J Cancer Res. 2023 Sep 15;13(9):4179-4191. eCollection 2023. Am J Cancer Res. 2023. PMID: 37818080 Free PMC article.
References
-
- Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384:1376–1388. - PubMed
-
- Tew WP, Fleming GF. Treatment of ovarian cancer in the older woman. Gynecol. Oncol. 2015;136:136–142. - PubMed
-
- Liu J, Matulonis UA. New strategies in ovarian cancer: translating the molecular complexity of ovarian cancer into treatment advances. Clin. Cancer Res. 2014;20:5150–5156. - PubMed
-
- Davis A, Tinker AV, Friedlander M. "Platinum resistant" ovarian cancer: what is it, who to treat and how to measure benefit? Gynecol. Oncol. 2014;133:624–631. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

