Beyond the passive interactions at the nano-bio interface: evidence of Cu metalloprotein-driven oxidative dissolution of silver nanoparticles
- PMID: 26801765
- PMCID: PMC4722631
- DOI: 10.1186/s12951-016-0160-6
Beyond the passive interactions at the nano-bio interface: evidence of Cu metalloprotein-driven oxidative dissolution of silver nanoparticles
Abstract
Background: In a biological system, an engineered nanomaterial (ENM) surface is altered by adsorbed proteins that modify ENM fate and toxicity. Thus far, protein corona characterizations have focused on protein adsorption, interaction strength, and downstream impacts on cell interactions. Given previous reports of Ag ENM disruption of Cu trafficking, this study focuses on Ag ENM interactions with a model Cu metalloprotein, Cu(II) azurin. The study provides evidence of otherwise overlooked ENM-protein chemical reactivity within the corona: redox activity.
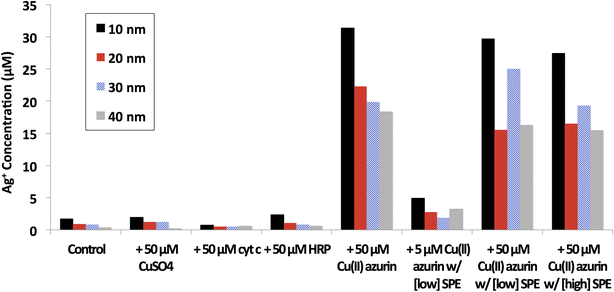
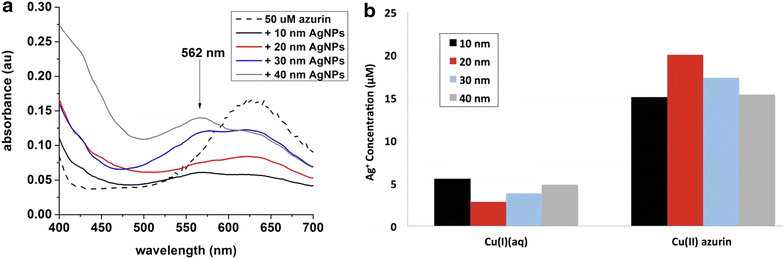
Results: Citrate-coated Ag ENMs of various sizes (10-40 nm) reacted with Cu(II) azurin resulted in an order of magnitude more dissolved ionic silver (Ag(I)(aq)) than samples of Ag ENMs only, ENMs mixed Cu(II) ions, or control proteins such as cytochrome c and horse radish peroxidase. This dramatic increase in ENM oxidative dissolution was observed even when Cu(II) azurin was combined with a diverse mixture of Escherchia coli proteins to mimic the complexity of the cellular conona. SDS PAGE results confirm that the multiprotein ENM corona includes azurin. A Cu(I)(aq) colorimetric indicator confirms Cu(II) azurin reduction upon interaction with Ag ENMs, but not with the addition of ionic silver, Ag(I)(aq).
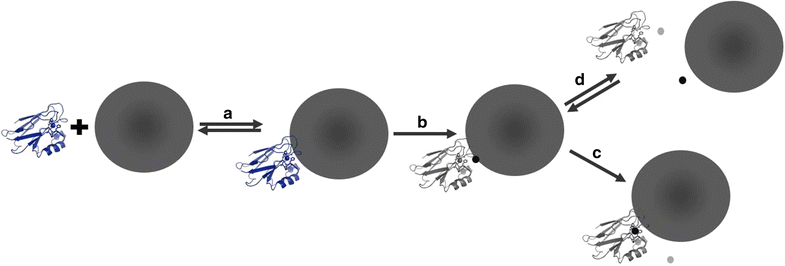
Conclusions: Cu(II) azurin and 10-40 nm Ag ENMs react to catalyze Ag ENM oxidative dissolution and reduction of the model Cu metalloprotein. Results push the current evaluation of protein-ENM characterization beyond passive binding interactions and enable the proposal of a mechanism for reactivity between a model Cu metalloprotein and Ag ENMs.
Figures
Similar articles
-
Structural and functional effects of Cu metalloprotein-driven silver nanoparticle dissolution.Environ Sci Technol. 2012 Jun 5;46(11):6355-62. doi: 10.1021/es300901h. Epub 2012 May 18. Environ Sci Technol. 2012. PMID: 22563882
-
Impacts of Pristine and Transformed Ag and Cu Engineered Nanomaterials on Surficial Sediment Microbial Communities Appear Short-Lived.Environ Sci Technol. 2016 Mar 1;50(5):2641-51. doi: 10.1021/acs.est.5b05054. Epub 2016 Feb 18. Environ Sci Technol. 2016. PMID: 26841726
-
Effect of Initial Speciation of Copper- and Silver-Based Nanoparticles on Their Long-Term Fate and Phytoavailability in Freshwater Wetland Mesocosms.Environ Sci Technol. 2017 Nov 7;51(21):12114-12122. doi: 10.1021/acs.est.7b02972. Epub 2017 Oct 23. Environ Sci Technol. 2017. PMID: 29017014
-
Ecophysiological perspectives on engineered nanomaterial toxicity in fish and crustaceans.Comp Biochem Physiol C Toxicol Pharmacol. 2017 Mar;193:30-41. doi: 10.1016/j.cbpc.2016.12.007. Epub 2016 Dec 23. Comp Biochem Physiol C Toxicol Pharmacol. 2017. PMID: 28017784 Review.
-
Physicochemical properties determine nanomaterial cellular uptake, transport, and fate.Acc Chem Res. 2013 Mar 19;46(3):622-31. doi: 10.1021/ar300031y. Epub 2012 Aug 14. Acc Chem Res. 2013. PMID: 22891796 Free PMC article. Review.
Cited by
-
Emerging investigator series: characterization of silver and silver nanoparticle interactions with zinc finger peptides.Environ Sci Nano. 2019 Aug 1;6(8):2367-2378. doi: 10.1039/C9EN00065H. Epub 2019 Jul 19. Environ Sci Nano. 2019. PMID: 31528351 Free PMC article.
-
Silver Nanoparticle Surface Chemistry Determines Interactions with Human Serum Albumin and Cytotoxic Responses in Human Liver Cells.ACS Omega. 2023 Jan 10;8(3):3310-3318. doi: 10.1021/acsomega.2c06882. eCollection 2023 Jan 24. ACS Omega. 2023. PMID: 36713725 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

