The Effectiveness of an Educational Brochure as a Risk Minimization Activity to Communicate Important Rare Adverse Events to Health-Care Professionals
- PMID: 26801772
- PMCID: PMC4769727
- DOI: 10.1007/s12325-016-0284-y
The Effectiveness of an Educational Brochure as a Risk Minimization Activity to Communicate Important Rare Adverse Events to Health-Care Professionals
Abstract
Introduction: Educational brochures are an important tool for communicating risk to health-care professionals. It is important to evaluate the impact of any risk minimization tool to understand the effectiveness of the strategy. The objective of this study was to assess the effectiveness (i.e., respondents' awareness and understanding of the communication) of a targeted educational brochure distributed to health-care professionals (HCPs) as a risk minimization strategy for the communication of new rare and important adverse events (AEs).
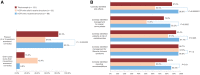
Methods: A prospective, non-interventional, online survey was performed following distribution of a specifically designed brochure highlighting new and important adverse events to a targeted HCP population, consisting of known users of the target medicine, as represented by a commercial database. Predefined multiple-choice survey questions assessed overall HCP awareness of the brochure and understanding and retention of information in those HCPs who reported receiving the brochure.
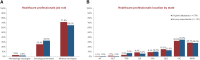
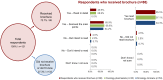
Results: The educational brochure was sent to a total of 565 HCPs; 121 (21.4%) responded to the survey. The majority of respondents (95.0%) had previously prescribed or dispensed the target medicine. In all, 88 (72.7%) respondents said they had received the educational brochure, of whom 95.5% stated they had at least scanned the main points. More participants who had received the brochure (86.4% to 96.6%) answered the five individual survey questions correctly compared with those who did not (51.5% to 97.0%); this was significant for four out of five questions (P ≤ 0.005). Significantly more HCPs who received the brochure achieved the predefined pass rate (at least four of five questions answered correctly) compared with HCPs who did not receive the brochure (93.2% vs 57.6%, respectively; P = 0.000003).
Conclusions: Distribution of targeted educational brochures may be an effective risk minimization strategy to raise HCP awareness of new rare and important AEs; educational brochures may also be an effective channel for sharing information on how these AEs can be best managed and on the importance and means of reporting AEs.
Funding: Celgene Pty Ltd, Melbourne, Australia.
Keywords: Abraxane; Australia; Brochure; Education; Health-care professionals; Nab-paclitaxel; Risk management plans; Risk minimization.
Figures
Similar articles
-
Evaluation of the Effectiveness of Additional Risk Minimization Measures for Voriconazole in the EU: Findings and Lessons Learned from a Healthcare Professional Survey.Pharmaceut Med. 2019 Apr;33(2):121-133. doi: 10.1007/s40290-019-00273-4. Pharmaceut Med. 2019. PMID: 31933256
-
Effectiveness Evaluation of Additional Risk Minimization Measures for Adolescent Use of Aripiprazole in the European Union: Results from a Post-Authorization Safety Study.Drug Saf. 2018 Aug;41(8):797-806. doi: 10.1007/s40264-018-0662-2. Drug Saf. 2018. PMID: 29671224 Free PMC article.
-
Use of Informational Brochures on Knowledge of Cataracts in Rural Ecuador.Cureus. 2023 Feb 27;15(2):e35555. doi: 10.7759/cureus.35555. eCollection 2023 Feb. Cureus. 2023. PMID: 37007411 Free PMC article.
-
Evaluation of patient reporting of adverse drug reactions to the UK 'Yellow Card Scheme': literature review, descriptive and qualitative analyses, and questionnaire surveys.Health Technol Assess. 2011 May;15(20):1-234, iii-iv. doi: 10.3310/hta15200. Health Technol Assess. 2011. PMID: 21545758 Review.
-
Knowledge, attitude, practice and barriers towards pharmacovigilance and adverse drug reactions reporting among healthcare professionals in Turkey: a systematic review.Curr Med Res Opin. 2022 Jan;38(1):145-154. doi: 10.1080/03007995.2021.1997287. Epub 2021 Nov 3. Curr Med Res Opin. 2022. PMID: 34694167
Cited by
-
Survery of healthcare professionals to assess the awareness, knowledge and self-reported behavior regarding recent fluoroquinolones safety issues.Med Pharm Rep. 2021 Oct;94(4):498-506. doi: 10.15386/mpr-1979. Epub 2021 Oct 30. Med Pharm Rep. 2021. PMID: 36105506 Free PMC article.
-
Whether Present Era Demand Change in Pharmaceutical Promotional Ways to be More Eco- and Doctor-Friendly? An Observational Study.J Pharm Bioallied Sci. 2024 Jul;16(Suppl 3):S2591-S2594. doi: 10.4103/jpbs.jpbs_247_24. Epub 2024 Jun 7. J Pharm Bioallied Sci. 2024. PMID: 39346399 Free PMC article.
-
Pharmacist-led educational interventions provided to healthcare providers to reduce medication errors: A systematic review and meta-analysis.PLoS One. 2021 Jun 23;16(6):e0253588. doi: 10.1371/journal.pone.0253588. eCollection 2021. PLoS One. 2021. PMID: 34161388 Free PMC article.
-
Understanding the Neuropsychological Implications of Klinefelter Syndrome in Pediatric Populations: Current Perspectives.Pediatr Rep. 2024 May 25;16(2):420-431. doi: 10.3390/pediatric16020036. Pediatr Rep. 2024. PMID: 38921701 Free PMC article. Review.
-
The RIMES Statement: A Checklist to Assess the Quality of Studies Evaluating Risk Minimization Programs for Medicinal Products.Drug Saf. 2018 Apr;41(4):389-401. doi: 10.1007/s40264-017-0619-x. Drug Saf. 2018. PMID: 29218682 Free PMC article.
References
-
- European Medicines Agency. Guideline on good pharmacovigilance practice (GVP). http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin.... Accessed 4 Nov 2015.
-
- Council for International Organization of Medical Sciences (CIOMS). Practical approaches to risk minimisation for medicinal products: report of CIOMS working group IX. http://www.cioms.ch/index.php/publications/available-publications/540/vi.... Accessed 4 Nov 2015.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

