Mechanisms of Action of Antiseizure Drugs and the Ketogenic Diet
- PMID: 26801895
- PMCID: PMC4852797
- DOI: 10.1101/cshperspect.a022780
Mechanisms of Action of Antiseizure Drugs and the Ketogenic Diet
Abstract
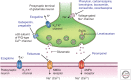
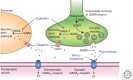
Antiseizure drugs (ASDs), also termed antiepileptic drugs, are the main form of symptomatic treatment for people with epilepsy, but not all patients become free of seizures. The ketogenic diet is one treatment option for drug-resistant patients. Both types of therapy exert their clinical effects through interactions with one or more of a diverse set of molecular targets in the brain. ASDs act by modulation of voltage-gated ion channels, including sodium, calcium, and potassium channels; by enhancement of γ-aminobutyric acid (GABA)-mediated inhibition through effects on GABAA receptors, the GABA transporter 1 (GAT1) GABA uptake transporter, or GABA transaminase; through interactions with elements of the synaptic release machinery, including synaptic vesicle 2A (SV2A) and α2δ; or by blockade of ionotropic glutamate receptors, including α-amino-3-hydroxy-5-methyl-4-isoxazole-propionate (AMPA) receptors. The ketogenic diet leads to increases in circulating ketones, which may contribute to the efficacy in treating pharmacoresistant seizures. Production in the brain of inhibitory mediators, such as adenosine, or ion channel modulators, such as polyunsaturated fatty acids, may also play a role. Metabolic effects, including diversion from glycolysis, are a further postulated mechanism. For some ASDs and the ketogenic diet, effects on multiple targets may contribute to activity. Better understanding of the ketogenic diet will inform the development of improved drug therapies to treat refractory seizures.
Copyright © 2016 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
References
-
- Avoli M, Kawasaki H, Zona C. 1996. Effects induced by topiramate on sodium electrogenesis in mammalian central neurons. Epilepsia 37: 51–52.
-
- Avoli M, Rogawski MA, Avanzini G. 2001. Generalized epileptic disorders: An update. Epilepsia 42: 445–457. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
