Pharmacogenetics driving personalized medicine: analysis of genetic polymorphisms related to breast cancer medications in Italian isolated populations
- PMID: 26801900
- PMCID: PMC4722680
- DOI: 10.1186/s12967-016-0778-z
Pharmacogenetics driving personalized medicine: analysis of genetic polymorphisms related to breast cancer medications in Italian isolated populations
Abstract
Background: Breast cancer is the most common cancer in women characterized by a high variable clinical outcome among individuals treated with equivalent regimens and novel targeted therapies. In this study, we performed a population based approach intersecting high-throughput genotype data from Friuli Venezia Giulia (FVG) isolated populations with publically available pharmacogenomics information to estimate the frequency of genotypes correlated with responsiveness to breast cancer treatment thus improving the clinical management of this disease in an efficient and cost effective way.
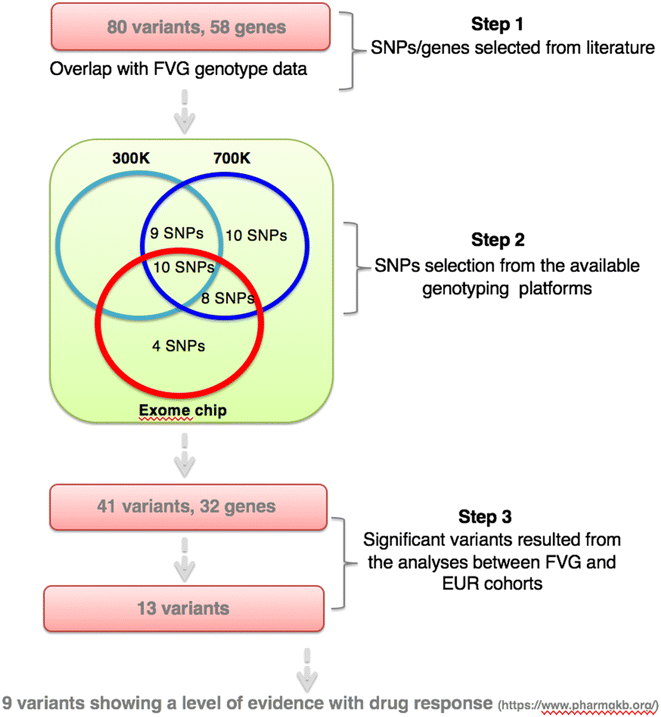
Methods: A list of 80 variants reported to be related to the efficacy or toxicity of breast cancer drugs was obtained from PharmGKB database. Fourty-one were present in FVG, 1000G European (EUR) and ExAC (Non Finnish European) databases. Their frequency was extracted using PLINK software and the differences tested by Fisher's exact test.
Results: Statistical analyses revealed that 13 out of the 41 (32 %) variants were significantly different in frequency in our sample as compared to the EUR/ExAC cohorts. For nine variants the available level of evidence (LOE) included polymorphisms related to cyclophosphamide, tamoxifen, doxorubicin, fluorpyrimidine and paclitaxel. In particular, for trastuzumab two variants were detected: (1) rs1801274-G within FCGR2A and associated with decreased efficacy (LOE 2B); (2) rs1136201-G located within ERBB2 and associated with increased toxicity (LOE 3). Both these two variants were underrepresented in the FVG population compared to EUR/ExAC population thus suggesting a high therapeutic index of this drug in our population. Moreover, as regards fluoropyrimidines, the frequency of two polymorphisms within the DPYD gene associated with drug toxicity (e.g., rs2297595-C allele and rs3918290-T allele, LOE 2A and 1, respectively) was extremely low in FVG population thus suggesting that a larger number of FVG patients could benefit from full dosage of fluoropyrimidine therapy.
Conclusions: All these findings increase the overall knowledge on the prevalence of specific variants related with breast cancer treatment responsiveness in FVG population and highlight the importance of assessing gene polymorphisms related with cancer medications in isolated communities.
Figures
Similar articles
-
Pharmacogenetics as Personalized Medicine: Association Investigation of SOD2 rs4880, CYP2C19 rs4244285, and FCGR2A rs1801274 Polymorphisms in a Breast Cancer Population in Iraqi Women.Clin Breast Cancer. 2018 Oct;18(5):e863-e868. doi: 10.1016/j.clbc.2018.01.009. Epub 2018 Jan 31. Clin Breast Cancer. 2018. PMID: 29482947
-
Dihydropyrimidine dehydrogenase pharmacogenetics for predicting fluoropyrimidine-related toxicity in the randomised, phase III adjuvant TOSCA trial in high-risk colon cancer patients.Br J Cancer. 2017 Oct 24;117(9):1269-1277. doi: 10.1038/bjc.2017.289. Epub 2017 Aug 24. Br J Cancer. 2017. PMID: 29065426 Free PMC article. Clinical Trial.
-
S4646 polymorphism in CYP19A1 gene is associated with the efficacy of hormone therapy in early breast cancer.Int J Clin Exp Pathol. 2015 May 1;8(5):5309-17. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26191232 Free PMC article.
-
Pharmacogenomics and breast cancer.Pharmacogenomics. 2004 Jan;5(1):31-55. doi: 10.1517/phgs.5.1.31.25686. Pharmacogenomics. 2004. PMID: 14683419 Review.
-
The role of pharmacogenetics in capecitabine efficacy and toxicity.Cancer Treat Rev. 2016 Nov;50:9-22. doi: 10.1016/j.ctrv.2016.08.001. Epub 2016 Aug 10. Cancer Treat Rev. 2016. PMID: 27569869 Review.
Cited by
-
Pharmacogenetics in Italy: current landscape and future prospects.Hum Genomics. 2024 Jul 10;18(1):78. doi: 10.1186/s40246-024-00612-w. Hum Genomics. 2024. PMID: 38987819 Free PMC article. Review.
-
Association of Single-Nucleotide Polymorphisms in Capecitabine Bioactivation Pathway with Adjuvant Therapy Safety in Colorectal Cancer Patients.Pharmaceutics. 2023 Oct 28;15(11):2548. doi: 10.3390/pharmaceutics15112548. Pharmaceutics. 2023. PMID: 38004528 Free PMC article.
-
Cyclophosphamide Pharmacogenomic Variation in Cancer Treatment and Its Effect on Bioactivation and Pharmacokinetics.Adv Pharmacol Pharm Sci. 2024 Jun 27;2024:4862706. doi: 10.1155/2024/4862706. eCollection 2024. Adv Pharmacol Pharm Sci. 2024. PMID: 38966316 Free PMC article. Review.
References
-
- Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M, Cutter D, Darby S, McGale P, Taylor C, Wang YC, Bergh J, Di Leo A, Albain K, Swain S, Piccart M, Pritchard K, Early Breast Cancer Trialists’ Collaborative Group(EBCTCG) Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432–444. doi: 10.1016/S0140-6736(11)61625-5. - DOI - PMC - PubMed
-
- Bedognetti D, Sertoli MR, Pronzato P, Del Mastro L, Venturini M, Taveggia P, Zanardi E, Siffredi G, Pastorino S, Queirolo P, Gardin G, Wang E, Monzeglio C, Boccardo F, Bruzzi P. Concurrent vs sequential adjuvant chemotherapy and hormone therapy in breast cancer: a multicenter randomized phase III trial. J Natl Cancer Inst. 2011;103:1529–1539. doi: 10.1093/jnci/djr351. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

