Dexketoprofen/tramadol 25 mg/75 mg: randomised double-blind trial in moderate-to-severe acute pain after abdominal hysterectomy
- PMID: 26801905
- PMCID: PMC4724087
- DOI: 10.1186/s12871-016-0174-5
Dexketoprofen/tramadol 25 mg/75 mg: randomised double-blind trial in moderate-to-severe acute pain after abdominal hysterectomy
Erratum in
-
Correction to: Dexketoprofen/tramadol 25 mg/75 mg: randomised double-blind trial in moderate-to-severe acute pain after abdominal hysterectomy.BMC Anesthesiol. 2017 Nov 30;17(1):159. doi: 10.1186/s12871-017-0452-x. BMC Anesthesiol. 2017. PMID: 29191204 Free PMC article.
Abstract
Background: Dexketoprofen trometamol plus tramadol hydrochloride is a new oral combination of two analgesics, which have different mechanisms of action for the treatment of moderate to severe acute pain.
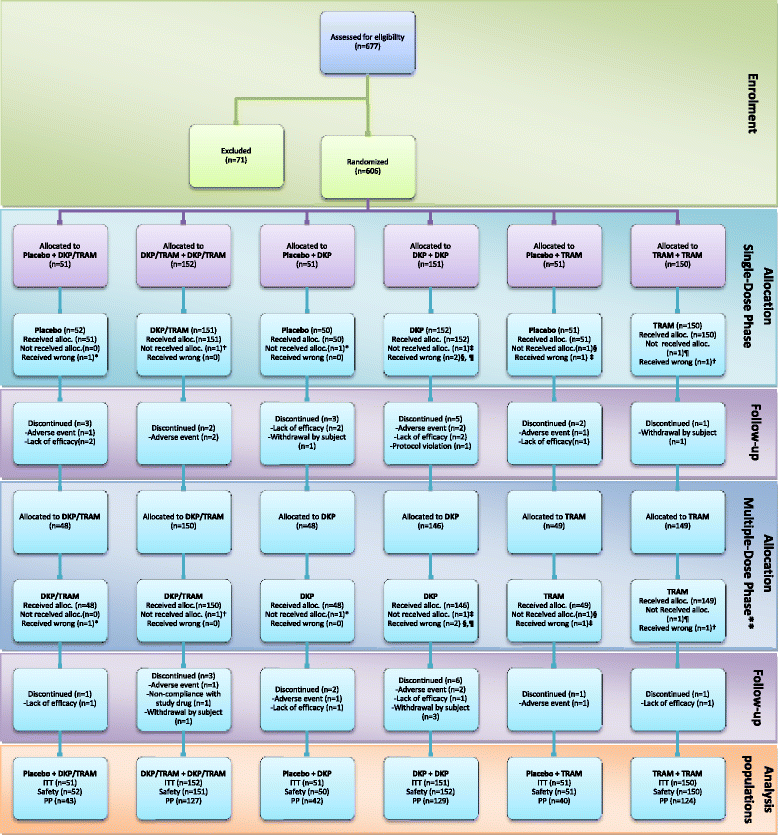
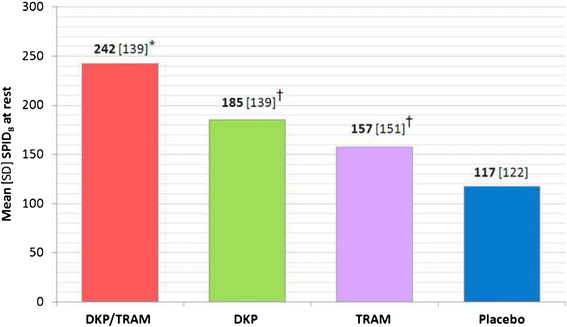
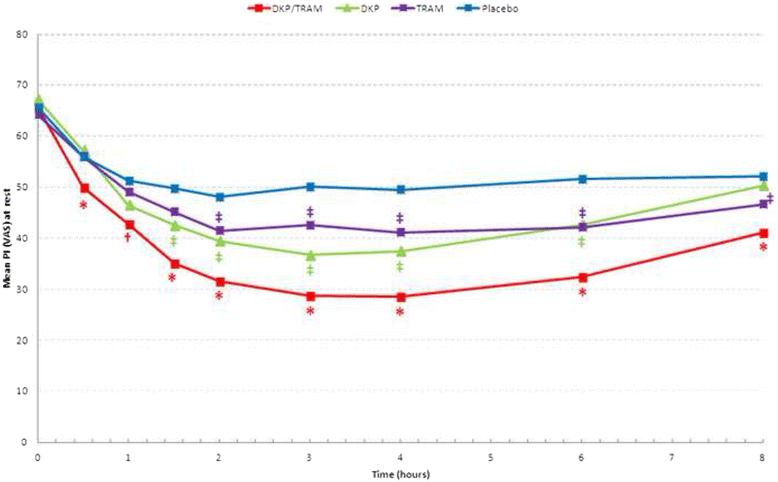
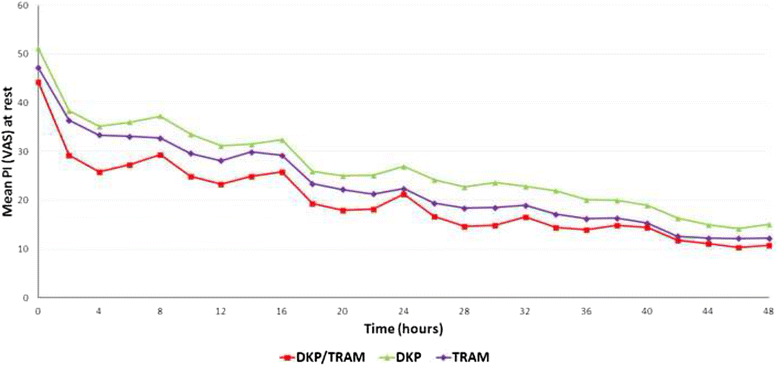
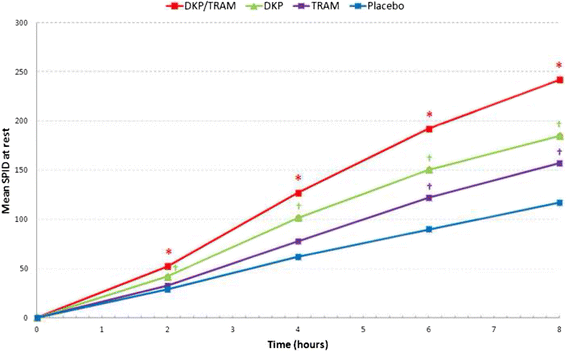
Methods: Randomised, double-blind, parallel, placebo and active-controlled, single and multiple-dose study to evaluate the analgesic efficacy and safety of dexketoprofen/tramadol 25 mg/75 mg in comparison with the single agents (dexketoprofen 25 mg and tramadol 100 mg) in moderate to severe acute pain after abdominal hysterectomy. Patients received seven consecutive doses of study drug within a 3-day period, each dose separated by an 8-hour interval. A placebo arm was included during the single-dose phase to validate the pain model. Efficacy assessments included pain intensity, pain relief, patient global evaluation and use of rescue medication. The primary endpoint was the mean sum of pain intensity differences over the first 8 h (SPID8).
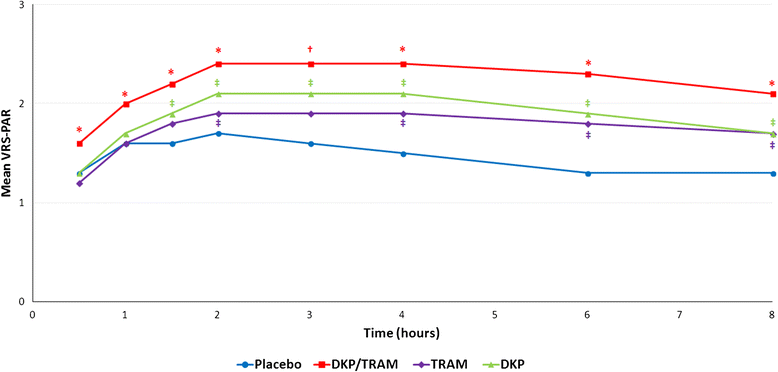
Results: The efficacy analysis included 606 patients, with a mean age of 48 years (range 25-73). The study results confirmed the superiority of the combination over the single agents in terms of the primary endpoint (p <0.001). Secondary endpoints were generally supportive of the superiority of the combination for both single and multiple doses. Most common adverse drug reactions (ADRs) were nausea (4.6%) and vomiting (2.3%). All other ADRs were experienced by less than 2% of patients.
Conclusions: The study results provided robust evidence of the superiority of dexketoprofen/tramadol 25 mg/75 mg over the single components in the management of moderate to severe acute pain, as confirmed by the single-dose efficacy, repeated-dose sustained effect and good safety profile observed.
Trial registration: EU Clinical Trials Register (EudraCT number 2012-004545-32, registered 04 October 2012); Clinicaltrials.gov ( NCT01904149, registered 17 July 2013).
Figures
References
-
- McGurk M, Robinson P, Rajayogeswaran V, De Luca M, Casini A, Artigas R, et al. Clinical comparison of dexketoprofen trometamol, ketoprofen, and placebo in postoperative dental pain. J Clin Pharmacol. 1998;38(12 Suppl):46S–54S. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

