Targeted antitumor prodrug therapy using CNGRC-yCD fusion protein in combination with 5-fluorocytosine
- PMID: 26801910
- PMCID: PMC4724154
- DOI: 10.1186/s12929-016-0227-6
Targeted antitumor prodrug therapy using CNGRC-yCD fusion protein in combination with 5-fluorocytosine
Abstract
Background: The enzyme-prodrug system is considered a promising tool for tumor treatment when conjugated with a targeting molecule. The asparagine-glycine-arginine (NGR) motif is a developing and interesting targeting peptide that could specifically bind to aminopeptidase N (APN), which is an NGR receptor expressed on the cell membrane of angiogenic endothelial cells and a number of tumor cells within the tumor tissues. The objective of this study was to develop a novel targeted enzyme-prodrug system using 5-fluorocytosine (5-FC) and an NGR-containing peptide fused with yeast cytosine deaminase (yCD), i.e. CNGRC-yCD fusion protein, to target APN-expressing cells within the tumor tissues and to convert 5-FC into 5-fluorouracil (5-FU) to kill tumors.
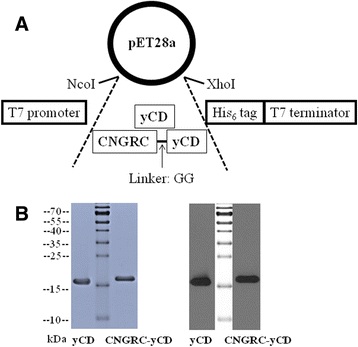
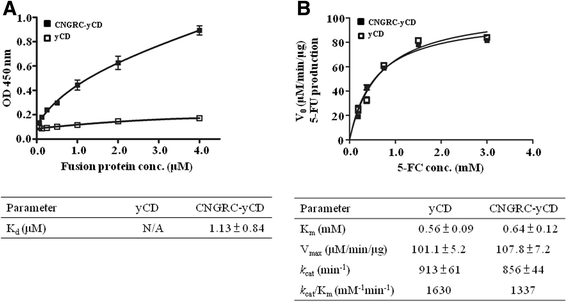
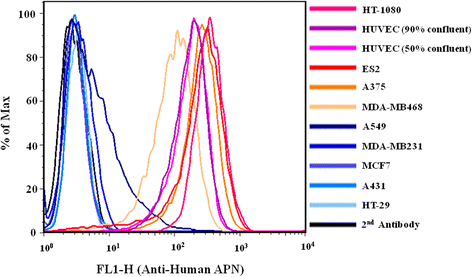
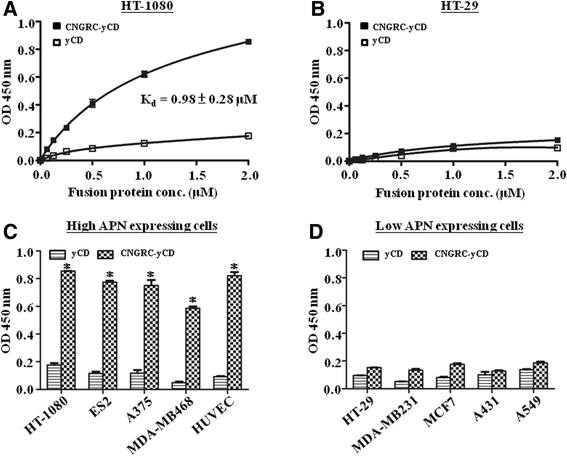
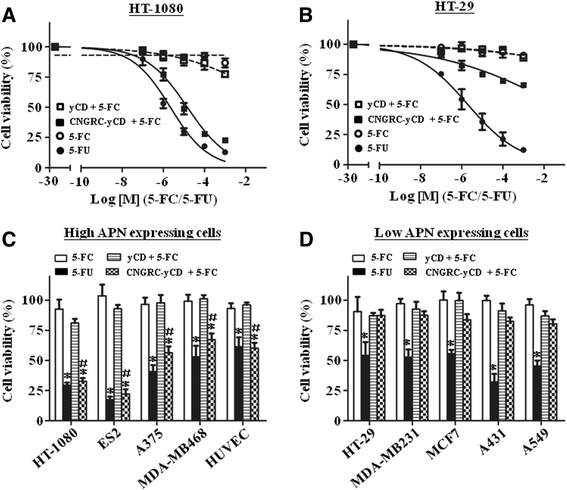
Results: Both yCD and CNGRC-yCD proteins were cloned into the pET28a vector and expressed by an Escherichia coli host. Both yCD and CNGRC-yCD proteins were purified and the yields were approximately 20 mg/L with over 95 % purity. The binding assay demonstrated that the CNGRC-yCD fusion protein had specific binding affinity toward purified APN recombinant protein and high-APN-expressing cells, including human endothelial cells (HUVECs) and various types of human tumor cell lines, but not low-APN-expressing tumor cell lines. Moreover, the enzyme activity and cell viability assay showed that the CNGRC-yCD fusion protein could effectively convert 5-FC into 5-FU and resulted in significant cell death in both high-APN-expressing tumor cells and HUVECs.
Conclusions: This study successfully constructs a new targeting enzyme-prodrug system, CNGRC-yCD fusion protein/5-FC. Systematic experiments demonstrated that the CNGRC-yCD protein retained both the APN-binding affinity of NGR and the enzyme activity of yCD to convert 5-FC into 5-FU. The combined treatment of the CNGRC-yCD protein with 5-FC resulted in the significantly increased cell death of high-APN-expressing cells as compared to that of low-APN-expressing cells.
Figures
Similar articles
-
Production of Long-Acting CNGRC-CPG2 Fusion Proteins: New Derivatives to Overcome Drug Immunogenicity of Ligand-Directed Enzyme Prodrug Therapy for Targeted Cancer Treatment.Technol Cancer Res Treat. 2021 Jan-Dec;20:15330338211057371. doi: 10.1177/15330338211057371. Technol Cancer Res Treat. 2021. PMID: 34802309 Free PMC article.
-
Transduction of yeast cytosine deaminase mediated by HIV-1 Tat basic domain into tumor cells induces chemosensitivity to 5-fluorocytosine.Exp Mol Med. 2004 Feb 29;36(1):43-51. doi: 10.1038/emm.2004.6. Exp Mol Med. 2004. PMID: 15031670
-
Yeast cytosine deaminase improves radiosensitization and bystander effect by 5-fluorocytosine of human colorectal cancer xenografts.Cancer Res. 2000 Dec 1;60(23):6649-55. Cancer Res. 2000. PMID: 11118048
-
Development of NGR-based anti-cancer agents for targeted therapeutics and imaging.Anticancer Agents Med Chem. 2012 Jan;12(1):76-86. doi: 10.2174/187152012798764714. Anticancer Agents Med Chem. 2012. PMID: 22023047 Review.
-
NGR-based strategies for targeting delivery of chemotherapeutics to tumor vasculature.Anticancer Agents Med Chem. 2012 Mar;12(3):239-46. doi: 10.2174/187152012800228751. Anticancer Agents Med Chem. 2012. PMID: 22263803 Review.
Cited by
-
Imaging Heterogeneous Patterns of Aminopeptidase N Activity in Hierarchical Tissue Structures Through High-Resolution Whole-Organ 3D Mapping.Angew Chem Int Ed Engl. 2025 May 26;64(22):e202504668. doi: 10.1002/anie.202504668. Epub 2025 Apr 14. Angew Chem Int Ed Engl. 2025. PMID: 40129052 Free PMC article.
-
Production of Long-Acting CNGRC-CPG2 Fusion Proteins: New Derivatives to Overcome Drug Immunogenicity of Ligand-Directed Enzyme Prodrug Therapy for Targeted Cancer Treatment.Technol Cancer Res Treat. 2021 Jan-Dec;20:15330338211057371. doi: 10.1177/15330338211057371. Technol Cancer Res Treat. 2021. PMID: 34802309 Free PMC article.
-
In vitro studies on CNGRC-CPG2 fusion proteins for ligand-directed enzyme prodrug therapy for targeted cancer therapy.Oncotarget. 2020 Feb 11;11(6):619-633. doi: 10.18632/oncotarget.27478. eCollection 2020 Feb 11. Oncotarget. 2020. PMID: 32110281 Free PMC article.
-
Engineering the Active Site Lid Dynamics to Improve the Catalytic Efficiency of Yeast Cytosine Deaminase.Int J Mol Sci. 2023 Apr 1;24(7):6592. doi: 10.3390/ijms24076592. Int J Mol Sci. 2023. PMID: 37047565 Free PMC article.
-
Gene-Directed Enzyme/Prodrug Therapy of Rat Brain Tumor Mediated by Human Mesenchymal Stem Cell Suicide Gene Extracellular Vesicles In Vitro and In Vivo.Cancers (Basel). 2022 Jan 31;14(3):735. doi: 10.3390/cancers14030735. Cancers (Basel). 2022. PMID: 35159002 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

