Selective localization of myosin-I proteins in macropinosomes and actin waves
- PMID: 26801966
- PMCID: PMC4769671
- DOI: 10.1002/cm.21275
Selective localization of myosin-I proteins in macropinosomes and actin waves
Abstract
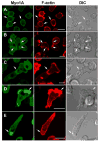
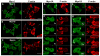
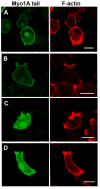
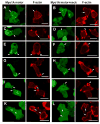
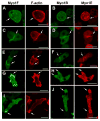
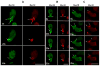
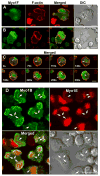
Class I myosins are widely expressed with roles in endocytosis and cell migration in a variety of cell types. Dictyostelium express multiple myosin Is, including three short-tailed (Myo1A, Myo1E, Myo1F) and three long-tailed (Myo1B, Myo1C, Myo1D). Here we report the molecular basis of the specific localizations of short-tailed Myo1A, Myo1E, and Myo1F compared to our previously determined localization of long-tailed Myo1B. Myo1A and Myo1B have common and unique localizations consistent with the various features of their tail region; specifically the BH sites in their tails are required for their association with the plasma membrane and heads are sufficient for relocalization to the front of polarized cells. Myo1A does not localize to actin waves and macropinocytic protrusions, in agreement with the absence of a tail region which is required for these localizations of Myo1B. However, in spite of the overall similarity of their domain structures, the cellular distributions of Myo1E and Myo1F are quite different from Myo1A. Myo1E and Myo1F, but not Myo1A, are associated with macropinocytic cups and actin waves. The localizations of Myo1E and Myo1F in macropinocytic structures and actin waves differ from the localization of Myo1B. Myo1B colocalizes with F-actin in the actin waves and at the tips of mature macropinocytic cups whereas Myo1E and Myo1F are in the interior of actin waves and along the entire surface of macropinocytic cups. Our results point to different mechanisms of targeting of short- and long-tailed myosin Is, and are consistent with these myosins having both shared and divergent cellular functions.
Keywords: BH site; actin waves; macropinocytosis; membrane binding; unconventional myosins.
© 2016 Wiley Periodicals, Inc.
Figures




Similar articles
-
Basic-hydrophobic sites are localized in conserved positions inside and outside of PH domains and affect localization of Dictyostelium myosin 1s.Mol Biol Cell. 2020 Jan 15;31(2):101-117. doi: 10.1091/mbc.E19-08-0475. Epub 2019 Nov 27. Mol Biol Cell. 2020. PMID: 31774725 Free PMC article.
-
Dictyostelium myosin 1F and myosin 1E inhibit actin waves in a lipid-binding-dependent and motor-independent manner.Cytoskeleton (Hoboken). 2020 Aug;77(8):295-302. doi: 10.1002/cm.21627. Cytoskeleton (Hoboken). 2020. PMID: 32734648 Free PMC article.
-
Molecular basis of dynamic relocalization of Dictyostelium myosin IB.J Biol Chem. 2012 Apr 27;287(18):14923-36. doi: 10.1074/jbc.M111.318667. Epub 2012 Feb 24. J Biol Chem. 2012. PMID: 22367211 Free PMC article.
-
Long-Tailed Unconventional Class I Myosins in Health and Disease.Int J Mol Sci. 2020 Apr 7;21(7):2555. doi: 10.3390/ijms21072555. Int J Mol Sci. 2020. PMID: 32272642 Free PMC article. Review.
-
Making cups and rings: the 'stalled-wave' model for macropinocytosis.Biochem Soc Trans. 2024 Aug 28;52(4):1785-1794. doi: 10.1042/BST20231426. Biochem Soc Trans. 2024. PMID: 38934501 Free PMC article. Review.
Cited by
-
MYO1B enhances colorectal cancer metastasis by promoting the F-actin rearrangement and focal adhesion assembly via RhoA/ROCK/FAK signaling.Ann Transl Med. 2021 Oct;9(20):1543. doi: 10.21037/atm-21-4702. Ann Transl Med. 2021. PMID: 34790749 Free PMC article.
-
Roles for 3' Phosphoinositides in Macropinocytosis.Subcell Biochem. 2022;98:119-141. doi: 10.1007/978-3-030-94004-1_7. Subcell Biochem. 2022. PMID: 35378706 Free PMC article.
-
Three-dimensional morphodynamic simulations of macropinocytic cups.iScience. 2021 Oct 1;24(10):103087. doi: 10.1016/j.isci.2021.103087. eCollection 2021 Oct 22. iScience. 2021. PMID: 34755081 Free PMC article.
-
A plasma membrane template for macropinocytic cups.Elife. 2016 Dec 13;5:e20085. doi: 10.7554/eLife.20085. Elife. 2016. PMID: 27960076 Free PMC article.
-
Formation and closure of macropinocytic cups in Dictyostelium.Curr Biol. 2023 Aug 7;33(15):3083-3096.e6. doi: 10.1016/j.cub.2023.06.017. Epub 2023 Jun 27. Curr Biol. 2023. PMID: 37379843 Free PMC article.
References
-
- Bagorda A, Mihaylov VA, Parent CA. Chemotaxis: moving forward and holding on to the past. Thromb Haemost. 2006;95:12–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

