Cell wall matrix polysaccharide distribution and cortical microtubule organization: two factors controlling mesophyll cell morphogenesis in land plants
- PMID: 26802013
- PMCID: PMC4765543
- DOI: 10.1093/aob/mcv187
Cell wall matrix polysaccharide distribution and cortical microtubule organization: two factors controlling mesophyll cell morphogenesis in land plants
Abstract
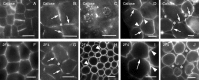
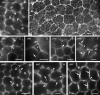
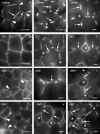
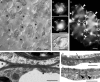
Background and aims: This work investigates the involvement of local differentiation of cell wall matrix polysaccharides and the role of microtubules in the morphogenesis of mesophyll cells (MCs) of three types (lobed, branched and palisade) in the dicotyledon Vigna sinensis and the fern Asplenium nidus.
Methods: Homogalacturonan (HGA) epitopes recognized by the 2F4, JIM5 and JIM7 antibodies and callose were immunolocalized in hand-made leaf sections. Callose was also stained with aniline blue. We studied microtubule organization by tubulin immunofluorescence and transmission electron microscopy.
Results: In both plants, the matrix cell wall polysaccharide distribution underwent definite changes during MC differentiation. Callose constantly defined the sites of MC contacts. The 2F4 HGA epitope in V. sinensis first appeared in MC contacts but gradually moved towards the cell wall regions facing the intercellular spaces, while in A. nidus it was initially localized at the cell walls delimiting the intercellular spaces, but finally shifted to MC contacts. In V. sinensis, the JIM5 and JIM7 HGA epitopes initially marked the cell walls delimiting the intercellular spaces and gradually shifted in MC contacts, while in A. nidus they constantly enriched MC contacts. In all MC types examined, the cortical microtubules played a crucial role in their morphogenesis. In particular, in palisade MCs, cortical microtubule helices, by controlling cellulose microfibril orientation, forced these MCs to acquire a truncated cone-like shape. Unexpectedly in V. sinensis, the differentiation of colchicine-affected MCs deviated completely, since they developed a cell wall ingrowth labyrinth, becoming transfer-like cells.
Conclusions: The results of this work and previous studies on Zea mays (Giannoutsou et al., Annals of Botany 2013; 112: : 1067-1081) revealed highly controlled local cell wall matrix differentiation in MCs of species belonging to different plant groups. This, in coordination with microtubule-dependent cellulose microfibril alignment, spatially controlled cell wall expansion, allowing MCs to acquire their particular shape.
Keywords: 2F4; Asplenium nidus; Callose; JIM5; JIM7; Vigna sinensis; cell contacts; cell wall; homogalacturonan.; microtubules; morphogenesis of photosynthetic cells; pectin epitopes.
© The Author 2016. Published by Oxford University Press on behalf of the Annals of Botany Company. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures







Similar articles
-
Early local differentiation of the cell wall matrix defines the contact sites in lobed mesophyll cells of Zea mays.Ann Bot. 2013 Oct;112(6):1067-81. doi: 10.1093/aob/mct175. Epub 2013 Aug 22. Ann Bot. 2013. PMID: 23969761 Free PMC article.
-
Local differentiation of cell wall matrix polysaccharides in sinuous pavement cells: its possible involvement in the flexibility of cell shape.Plant Biol (Stuttg). 2018 Mar;20(2):223-237. doi: 10.1111/plb.12681. Epub 2018 Jan 10. Plant Biol (Stuttg). 2018. PMID: 29247575
-
Callose and homogalacturonan epitope distribution in stomatal complexes of Zea mays and Vigna sinensis.Protoplasma. 2020 Jan;257(1):141-156. doi: 10.1007/s00709-019-01425-8. Epub 2019 Aug 30. Protoplasma. 2020. PMID: 31471650
-
Callose: a multifunctional (1, 3)-β-D-glucan involved in morphogenesis and function of angiosperm stomata.J Biol Res (Thessalon). 2021 Aug 3;28(1):17. doi: 10.1186/s40709-021-00150-9. J Biol Res (Thessalon). 2021. PMID: 34344461 Free PMC article. Review.
-
Cellulose and callose synthesis and organization in focus, what's new?Curr Opin Plant Biol. 2016 Dec;34:9-16. doi: 10.1016/j.pbi.2016.07.007. Epub 2016 Jul 29. Curr Opin Plant Biol. 2016. PMID: 27479608 Review.
Cited by
-
A belt for the cell: cellulosic wall thickenings and their role in morphogenesis of the 3D puzzle cells in walnut shells.J Exp Bot. 2021 Jun 22;72(13):4744-4756. doi: 10.1093/jxb/erab197. J Exp Bot. 2021. PMID: 33963747 Free PMC article.
-
Spatio-temporal diversification of the cell wall matrix materials in the developing stomatal complexes of Zea mays.Planta. 2016 Nov;244(5):1125-1143. doi: 10.1007/s00425-016-2574-7. Epub 2016 Jul 26. Planta. 2016. PMID: 27460945
-
Nutritive tissue rich in reserves in the cell wall and protoplast: the case of Manihot esculenta (Euphorbiaceae) galls induced by Iatrophobia brasiliensis (Diptera, Cecidomyiidae).Protoplasma. 2024 May;261(3):513-525. doi: 10.1007/s00709-023-01912-z. Epub 2023 Dec 19. Protoplasma. 2024. PMID: 38114665
-
Chemical composition of cell wall changes during developmental stages of galls on Matayba guianensis (Sapindaceae): perspectives obtained by immunocytochemistry analysis.Naturwissenschaften. 2021 Apr 19;108(3):16. doi: 10.1007/s00114-021-01732-2. Naturwissenschaften. 2021. PMID: 33871712
-
Cortical Microtubule Organization during Petal Morphogenesis in Arabidopsis.Int J Mol Sci. 2019 Oct 3;20(19):4913. doi: 10.3390/ijms20194913. Int J Mol Sci. 2019. PMID: 31623377 Free PMC article. Review.
References
-
- Altartouri Β, Geitmann Α. 2014. Understanding plant cell morphogenesis requires real-time monitoring of cell wall polymers. Current Opinion in Plant Biology 23C: 76–82. - PubMed
-
- Apostolakos P, Galatis B, Katsaros C, Schnepf E. 1990. Tubulin conformation in microtubule-free cells of Vigna sinensis: an immunofluorescent and electron microscope study. Protoplasma 154: 132–143.
-
- Apostolakos P, Galatis B, Panteris E. 1991. Microtubules in cell morphogenesis and intercellular space formation in Zea mays leaf mesophyll and Pilea cadierei epithem. Journal of Plant Physiology 137: 591–601.
-
- Bacic A, Fincher GB, Stone BA. 2009. Chemistry, biochemistry, and biology of (1→3)-β-glucans and related polysaccharides. Amsterdam: Academic Press.
-
- Baluška F, Liners F, Hlavačka A, et al. 2005. Cell wall pectins and xyloglucans are internalized into dividing root cells and accumulate within cell plates during cytokinesis. Protoplasma 225: 141–155. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

