Cognition and neuropsychiatry in behavioral variant frontotemporal dementia by disease stage
- PMID: 26802093
- PMCID: PMC4762418
- DOI: 10.1212/WNL.0000000000002373
Cognition and neuropsychiatry in behavioral variant frontotemporal dementia by disease stage
Abstract
Objective: To characterize the cognitive and neuropsychiatric symptoms of patients with behavioral variant frontotemporal dementia (bvFTD) over the natural course of the disease.
Methods: We examined the initial and subsequent neuropsychological test performance and neuropsychiatric symptoms in a large cohort of patients with bvFTD (n = 204) across progressive stages of disease as measured by the Clinical Dementia Rating (CDR). We also compared cognitive and neuropsychiatric impairments of patients with bvFTD to those of an age-matched cohort with Alzheimer disease (AD) dementia (n = 674).
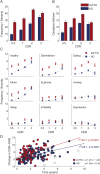
Results: At the earliest stage (CDR = 0.5), patients with bvFTD had profound neuropsychiatric disturbances, insensitivity to errors, slower response times, and poor naming, with intact attention span, memory, and facial affect naming. Tests continuing to show progressive, statistically significant stepwise declines after the CDR = 1 stage included free recall, visuoconstruction, set-shifting, error insensitivity, semantic fluency, design fluency, emotion naming, calculations, confrontation naming, syntax comprehension, and verbal agility. At CDR = 0.5, patients with bvFTD significantly outperformed patients with AD in episodic memory and were faster in set-shifting, while scoring quantitatively worse in lexical fluency, emotion naming, and error sensitivity. The overall rate of disease progression in bvFTD was more rapid than in AD.
Conclusion: There are distinct patterns of cognitive deficits differentiating the earlier and later disease stages in bvFTD, with the pattern of cognitive decline revealing in greater detail the natural history of the disease. These cognitive symptoms are readily apparent clinical markers of dysfunction in the principal brain networks known to undergo molecular and anatomical changes in bvFTD, thus are important indicators of the evolving pathology in individual patients.
© 2016 American Academy of Neurology.
Figures


Comment in
-
Cognition and neuropsychiatry in behavioral variant frontotemporal dementia by disease stage.Neurology. 2016 Oct 4;87(14):1523. doi: 10.1212/01.wnl.0000503343.29930.76. Neurology. 2016. PMID: 27698154 Free PMC article. No abstract available.
References
-
- Kramer JH, Jurik J, Sha SJ, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol 2003;16:211–218. - PubMed
Publication types
MeSH terms
Grants and funding
- K23 AG045289/AG/NIA NIH HHS/United States
- P50 AG023501/AG/NIA NIH HHS/United States
- K23AG021606/AG/NIA NIH HHS/United States
- K23 AG040127/AG/NIA NIH HHS/United States
- K24 AG045333/AG/NIA NIH HHS/United States
- K23AG045289/AG/NIA NIH HHS/United States
- RF1 AG029577/AG/NIA NIH HHS/United States
- P01 AG017586/AG/NIA NIH HHS/United States
- K23 AG038357/AG/NIA NIH HHS/United States
- R01 AG038791/AG/NIA NIH HHS/United States
- K23 AG048291/AG/NIA NIH HHS/United States
- K23 AG042492-01/AG/NIA NIH HHS/United States
- P01 AG019724/AG/NIA NIH HHS/United States
- P50AG02350/AG/NIA NIH HHS/United States
- R01 AG032306/AG/NIA NIH HHS/United States
- K23 AG039414/AG/NIA NIH HHS/United States
- K23 AG037566/AG/NIA NIH HHS/United States
- K23 AG042492/AG/NIA NIH HHS/United States
- K23 AG021606/AG/NIA NIH HHS/United States
- R01AG029577/AG/NIA NIH HHS/United States
- K23AG040127/AG/NIA NIH HHS/United States
- U54 NS092089/NS/NINDS NIH HHS/United States
- P01AG019724/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
