Nephrin Tyrosine Phosphorylation Is Required to Stabilize and Restore Podocyte Foot Process Architecture
- PMID: 26802179
- PMCID: PMC4978059
- DOI: 10.1681/ASN.2015091048
Nephrin Tyrosine Phosphorylation Is Required to Stabilize and Restore Podocyte Foot Process Architecture
Abstract
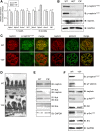
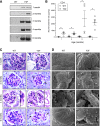
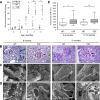
Podocytes are specialized epithelial cells of the kidney blood filtration barrier that contribute to permselectivity via a series of interdigitating actin-rich foot processes. Positioned between adjacent projections is a unique cell junction known as the slit diaphragm, which is physically connected to the actin cytoskeleton via the transmembrane protein nephrin. Evidence indicates that tyrosine phosphorylation of the intracellular tail of nephrin initiates signaling events, including recruitment of cytoplasmic adaptor proteins Nck1 and Nck2 that regulate actin cytoskeletal dynamics. Nephrin tyrosine phosphorylation is altered in human and experimental renal diseases characterized by pathologic foot process remodeling, prompting the hypothesis that phosphonephrin signaling directly influences podocyte morphology. To explore this possibility, we generated and analyzed knockin mice with mutations that disrupt nephrin tyrosine phosphorylation and Nck1/2 binding (nephrin(Y3F/Y3F) mice). Homozygous nephrin(Y3F/Y3F) mice developed progressive proteinuria accompanied by structural changes in the filtration barrier, including podocyte foot process effacement, irregular thickening of the glomerular basement membrane, and dilated capillary loops, with a similar but later onset phenotype in heterozygous animals. Furthermore, compared with wild-type mice, nephrin(Y3F/Y3F) mice displayed delayed recovery in podocyte injury models. Profiling of nephrin tyrosine phosphorylation dynamics in wild-type mice subjected to podocyte injury indicated site-specific differences in phosphorylation at baseline, injury, and recovery, which correlated with loss of nephrin-Nck1/2 association during foot process effacement. Our results define an essential requirement for nephrin tyrosine phosphorylation in stabilizing podocyte morphology and suggest a model in which dynamic changes in phosphotyrosine-based signaling confer plasticity to the podocyte actin cytoskeleton.
Keywords: cell biology and structure; cell signaling; cytoskeleton; glomerular disease; nephrin; podocyte.
Copyright © 2016 by the American Society of Nephrology.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

