Unusual flavoenzyme catalysis in marine bacteria
- PMID: 26803009
- PMCID: PMC4870101
- DOI: 10.1016/j.cbpa.2016.01.001
Unusual flavoenzyme catalysis in marine bacteria
Abstract
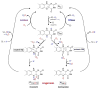
Ever since the discovery of the flavin cofactor more than 80 years ago, flavin-dependent enzymes have emerged as ubiquitous and versatile redox catalysts in primary metabolism. Yet, the recent advances in the discovery and characterization of secondary metabolic pathways exposed new roles for flavin-mediated catalysis in the generation of structurally complex natural products. Here, we review a selection of key biosynthetic flavoenzymes from marine bacterial secondary metabolism and illustrate how their functional and mechanistic investigation expanded our view of the cofactor's chemical repertoire and led to the discovery of a previously unknown flavin redox state.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures



References
-
- Molinski TF, Dalisay DS, Lievens SL, Saludes JP. Drug development from marine natural products. Nat Rev Drug Discov. 2009;8:69–85. - PubMed
-
- Blunt JW, Copp BR, Keyzers RA, Munro MHG, Prinsep MR. Marine natural products. Nat Prod Rep. 2015;32:116–211. - PubMed
-
- Piel J. Metabolites from symbiotic bacteria. Nat Prod Rep. 2009;26:338–362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

