VEGF-A acts via neuropilin-1 to enhance epidermal cancer stem cell survival and formation of aggressive and highly vascularized tumors
- PMID: 26804163
- PMCID: PMC4959993
- DOI: 10.1038/onc.2015.507
VEGF-A acts via neuropilin-1 to enhance epidermal cancer stem cell survival and formation of aggressive and highly vascularized tumors
Abstract
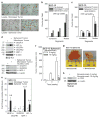
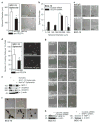
We identify a limited subpopulation of epidermal cancer stem cells (ECS cells), in squamous cell carcinoma, that form rapidly growing, invasive and highly vascularized tumors, as compared with non-stem cancer cells. These ECS cells grow as non-attached spheroids, and display enhanced migration and invasion. We show that ECS cell-produced vascular endothelial growth factor (VEGF)-A is required for the maintenance of this phenotype, as knockdown of VEGF-A gene expression or treatment with VEGF-A-inactivating antibody reduces these responses. In addition, treatment with bevacizumab reduces tumor vascularity and growth. Surprisingly, the classical mechanism of VEGF-A action via interaction with VEGF receptors does not mediate these events, as these cells lack VEGFR1 and VEGFR2. Instead, VEGF-A acts via the neuropilin-1 (NRP-1) co-receptor. Knockdown of NRP-1 inhibits ECS cell spheroid formation, invasion and migration, and attenuates tumor formation. These studies suggest that VEGF-A acts via interaction with NRP-1 to trigger intracellular events leading to ECS cell survival and formation of aggressive, invasive and highly vascularized tumors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- American Cancer Society Website. Cancer Facts and Figures. 2010 Available at: http://www.cancer.org/
-
- Bickers DR, Lim HW, Margolis D, Weinstock MA, Goodman C, Faulkner E, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490–500. - PubMed
-
- Rogers HW, Weinstock MA, Harris AR, Hinckley MR, Feldman SR, Fleischer AB, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283–287. - PubMed
-
- Folkman J. The role of angiogenesis in tumor growth. Semin Cancer Biol. 1992;3:65–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

