[Effect and Mechanism of Radiosensitization of Poly (ADP-Ribose) Polymerase Inhibitor n Lewis Cells and Xenografts]
- PMID: 26805733
- PMCID: PMC5999804
- DOI: 10.3779/j.issn.1009-3419.2016.01.02
[Effect and Mechanism of Radiosensitization of Poly (ADP-Ribose) Polymerase Inhibitor n Lewis Cells and Xenografts]
Abstract
Background and objective: The DNA damage of the irradiated tumor cells is mainly single strand breaks (SSBs) and double strand breaks (DSBs), in which the frequency of occurrence of SSBs is dozens of times than DSBs. However, most of the SSBs could be repaired by the Poly (ADP-Ribose) Polymerase (PARP) and other related factors. Recently listed drug-Olaparib (PARP1/PARP2/PARP3 inhibitor) could target the repair pathways of single strand breaks, and recent clinical trials of PARP inhibitors combined with chemotherapy obtained encouraging results. The aim of this study is to investigate the effect and potential mechanism of radiosensitization of Poly (ADP-Ribose) polymerase inhibitor-Olaparib on lewis cells and xenografts.
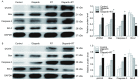
Methods: The inhibition concentration 10% inhibitory concentration (IC10) of Olaparib to Lewis cells was detected by methyl thiazolyltetrazolium (MTT) assay. The radiosensitization effect of Olaparib on Lewis cells was determined by classical colony forming assay. Lewis xenografts models were established, and the mice were randomly divided into four groups: Control group, Olaparib group, Radiotherapy group (RT, 2 Gy × 5 d), Olaparib combined with RT group. Xenograft volume was measured during the treatment. Flow cytometry was used to analyze the apoptosis rate of the Lewis cells in each group, and the apoptosis of xenograft tissues was observed by TUNEL stain. The ralative protein levels of γH2AX (associated with DNA strand breaks repair), Bax/Bcl-2, Caspase-3 (apoptosis-associated protein) were detected by Western blot in vitro and in vivo.
Results: The IC10 value of Olaparib was 4.4 μmol/L. The radio-sensitivity enhancement ratio (SER) of Olaparib combined with RT was 1.211 in vitro. Compared with RT (2 Gy × 5 d) alone, the combination of Olaparib with fractionated radiotherapy significantly increased the growth delay of Lewis xenografts (P<0.001). Flow cytometry and TUNEL analysis indicated that the apoptosis rate in the combination group was significantly higher than in RT group in vitro and in vivo (P<0.05). Furthermore, Western blot results confirmed that in the combination group the expression levels of γH2AX, Bax, Caspase-3 were increased, while that of Bcl-2 was decreased as opposed to RT group (P<0.05).
Conclusion: The combination of Olaprib and fractionated radiotherapy can markedly improve the radiobiological effects on lewis cells and xenografts, which may be induced by promoting the formation of DNA double strand break and upregulating the expression of Bax/Bcl-2 pro-apoptotic proteins.
背景与目的 受电离辐射的肿瘤细胞DNA损伤主要为单链断裂(single strand break, SSB)与双链断裂(double strand break, DSB),其中SSBs发生的频率数十倍于DSBs,而SSBs多能通过聚腺苷二磷酸核糖聚合酶[Poly(ADP-Ribose) polymerase, PARP]等因子进行修复。相关新药Olaparib(PARP1/PARP2/PARP3抑制剂)靶向作用于细胞SSBs损伤修复,其联合化疗的临床研究取得令人鼓舞结果。本实验旨在研究Olaparib对Lewis肺癌细胞及移植瘤放疗增敏作用,初步探讨其可能机制。方法 采用MTT法检测Olaparib对Lewis细胞10%抑制浓度(10% inhibitory concentration, IC10)值,克隆形成实验验证Olaparib联合放疗的体外增敏作用;成瘤小鼠分为空白对照、Olaparib、放疗(radiotherapy, RT, 2 Gy×5 d)、Olaparib+RT组,动态测量各组移植瘤体积变化;流式细胞术比较各组细胞体外凋亡率,TUNEL法比较移植瘤细胞凋亡;Western blot检测各组DNA损伤相关蛋白γH2AX,凋亡相关蛋白Bax/Bcl-2、Caspase-3表达。结果 Olaparib对Lewis细胞IC10值为4.4 μmol/L,克隆形成实验测得Olaparib放疗增敏比为1.211;移植瘤初体积(处理前)增长4倍所需天数,Olaparib+RT组显著高于单纯RT组(P<0.001);流式及TUNEL法检测Lewis细胞体内外凋亡率均Olaparib+RT组高于RT组(P<0.05);Olaparib+RT组细胞及移植瘤中γH2AX、Bax、Caspase-3显著高于RT组,Bcl-2显著低于RT组(均P<0.05)。结论 Olaparib对Lewis肺癌细胞及移植瘤起到显著放疗增敏作用,其机制可能与增加受照肿瘤细胞DNA双链断裂形成,上调Bax/Bcl-2促凋亡体系蛋白有关。.
Figures




References
-
-
Yin WB, Yu ZH, Xu GZ. Radiation oncology. 4nd ed. Beijing (China): Pecking Union Medical College Press; 2008. Chapitre 3, radiobiology. 231-458.
-
殷蔚伯, 余子豪, 徐国镇. 肿瘤放射治疗学. 第4版, 北京: 中国协和医科大学出版社; 2008. 第三篇, 放射生物学. 231-458.
-
-
- Shrivastav M, De Haro LP, Nickoloff JA. Regulation of DNA double-strand break repair pathway choice. https://academic.oup.com/nar. Cell Res. 2008;18(1):134–147. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

