NHC Backbone Configuration in Ruthenium-Catalyzed Olefin Metathesis
- PMID: 26805793
- PMCID: PMC6274038
- DOI: 10.3390/molecules21010117
NHC Backbone Configuration in Ruthenium-Catalyzed Olefin Metathesis
Abstract
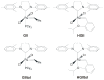
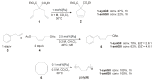
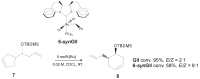
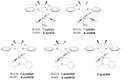
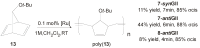
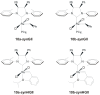
The catalytic properties of olefin metathesis ruthenium complexes bearing N-heterocyclic carbene ligands with stereogenic centers on the backbone are described. Differences in catalytic behavior depending on the backbone configurations of symmetrical and unsymmetrical NHCs are discussed. In addition, an overview on asymmetric olefin metathesis promoted by chiral catalysts bearing C₂-symmetric and C₁-symmetric NHCs is provided.
Keywords: NHC ligands; metathesis; ruthenium catalysts; stereogenic centers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
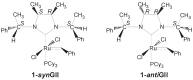


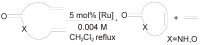

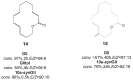
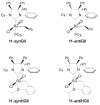

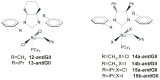
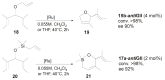
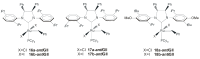


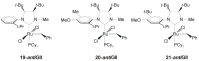
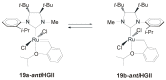
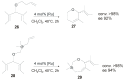


References
-
- Grubbs R.H. Handbook of Metathesis. Volume 1–3 Wiley-VCH; Weinheim, Germany: 2003.
-
- Connon S.J., Blechert S. In: Ruthenium Catalysts and Fine Chemistry. Dixneuf P.H., Bruneau C., editors. Volume 11. Springer; Heidelberg, Germany: 2004. pp. 93–124.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

