Cancer Immunosurveillance by Tissue-Resident Innate Lymphoid Cells and Innate-like T Cells
- PMID: 26806130
- PMCID: PMC4733424
- DOI: 10.1016/j.cell.2016.01.002
Cancer Immunosurveillance by Tissue-Resident Innate Lymphoid Cells and Innate-like T Cells
Abstract
Malignancy can be suppressed by the immune system in a process termed immunosurveillance. However, to what extent immunosurveillance occurs in spontaneous cancers and the composition of participating cell types remains obscure. Here, we show that cell transformation triggers a tissue-resident lymphocyte response in oncogene-induced murine cancer models. Non-circulating cytotoxic lymphocytes, derived from innate, T cell receptor (TCR)αβ, and TCRγδ lineages, expand in early tumors. Characterized by high expression of NK1.1, CD49a, and CD103, these cells share a gene-expression signature distinct from those of conventional NK cells, T cells, and invariant NKT cells. Generation of these lymphocytes is dependent on the cytokine IL-15, but not the transcription factor Nfil3 that is required for the differentiation of tumor-infiltrating NK cells, and IL-15 deficiency, but not Nfil3 deficiency, results in accelerated tumor growth. These findings reveal a tumor-elicited immunosurveillance mechanism that engages unconventional type-1-like innate lymphoid cells and type 1 innate-like T cells.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


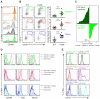
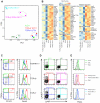

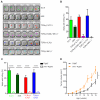
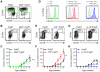
Comment in
-
Tumour immunology: Innate surveillance.Nat Rev Immunol. 2016 Mar;16(3):132-3. doi: 10.1038/nri.2016.20. Epub 2016 Feb 15. Nat Rev Immunol. 2016. PMID: 26875829 No abstract available.
-
Tissue-resident lymphocytes: sentinel of the transformed tissue.J Immunother Cancer. 2017 May 16;5:41. doi: 10.1186/s40425-017-0244-3. eCollection 2017. J Immunother Cancer. 2017. PMID: 28515941 Free PMC article.
References
-
- Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. - PubMed
-
- Bernink JH, Krabbendam L, Germar K, de Jong E, Gronke K, Kofoed-Nielsen M, Munneke JM, Hazenberg MD, Villaudy J, Buskens CJ, et al. Interleukin-12 and -23 Control Plasticity of CD127(+) Group 1 and Group 3 Innate Lymphoid Cells in the Intestinal Lamina Propria. Immunity. 2015;43:146–160. - PubMed
-
- Bernink JH, Peters CP, Munneke M, te Velde AA, Meijer SL, Weijer K, Hreggvidsdottir HS, Heinsbroek SE, Legrand N, Buskens CJ, et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nature immunology. 2013;14:221–229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

