Assessment of neonatal platelet adhesion, activation, and aggregation
- PMID: 26806373
- PMCID: PMC4828266
- DOI: 10.1111/jth.13270
Assessment of neonatal platelet adhesion, activation, and aggregation
Abstract
Background: Acquired and inherited bleeding disorders may present in the neonatal period with devastating lifelong effects. Diagnosing bleeding disorders in the neonatal population could aid in preventing and treating the associated complications. However, currently available platelet function testing is limited in neonates, owing to difficulties in obtaining an adequate blood volume, a lack of normal reference ranges, and an incomplete understanding of the neonatal platelet functional phenotype.
Objective: To develop small-volume, whole blood platelet function assays in order to quantify and compare neonatal and adult platelet function.
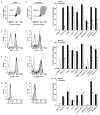
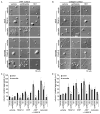
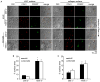
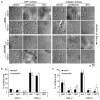
Methods and results: Peripheral blood was obtained from healthy, full-term neonates at 24 h of life. Platelet activation, secretion and aggregation were measured via flow cytometry. Platelet adhesion and aggregation were assessed under static and flow conditions. As compared with adult platelets, peripheral neonatal platelet P-selectin expression and integrin glycoprotein IIbIIIa activation were significantly reduced in response to the G-protein-coupled receptor (GPCR) agonists thrombin receptor activator peptide-6 (TRAP-6), ADP, and U46619, and the immunoreceptor tyrosine-based activation motif (ITAM) signaling pathway agonists collagen-related peptide (CRP) and rhodocytin. Neonatal platelet aggregation was markedly reduced in response to TRAP-6, ADP, U46619, CRP and rhodocytin as compared with adult platelets. The extents of neonatal and adult platelet adhesion and aggregate formation under static and shear conditions on collagen and von Willebrand factor were similar.
Conclusions: As compared with adult platelets, we found that neonatal platelet activation and secretion were blunted in response to GPCR or ITAM agonists, whereas the extent of neonatal platelet adhesion and aggregate formation was similar to that of adult platelets.
Keywords: neonate; platelet activation; platelet adhesiveness; platelet aggregation; platelet function tests.
© 2016 International Society on Thrombosis and Haemostasis.
Conflict of interest statement
The authors state that they have no conflict of interest.
Figures


References
-
- Wei AH, Schoenwaelder SM, Andrews RK, Jackson SP. New insights into the haemostatic function of platelets. Br J Haematol. 2009;147:415–30. - PubMed
-
- Del Vecchio A, Motta M, Romagnoli C. Neonatal platelet function. Clin Perinatol. 2015;42:625–38. - PubMed
-
- Kühne T, Imbach P. Neonatal platelet physiology and pathophysiology. Eur J Pediatr. 1998;157:87–94. - PubMed
-
- Andrew M, Paes B, Milner R, Johnston M, Mitchell L, Tollefsen DM, Powers P. Development of the human coagulation system in the full-term infant. Blood. 1987;70:165–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

