Genetic variants associated with response to lithium treatment in bipolar disorder: a genome-wide association study
- PMID: 26806518
- PMCID: PMC4814312
- DOI: 10.1016/S0140-6736(16)00143-4
Genetic variants associated with response to lithium treatment in bipolar disorder: a genome-wide association study
Abstract
Background: Lithium is a first-line treatment in bipolar disorder, but individual response is variable. Previous studies have suggested that lithium response is a heritable trait. However, no genetic markers of treatment response have been reproducibly identified.
Methods: Here, we report the results of a genome-wide association study of lithium response in 2563 patients collected by 22 participating sites from the International Consortium on Lithium Genetics (ConLiGen). Data from common single nucleotide polymorphisms (SNPs) were tested for association with categorical and continuous ratings of lithium response. Lithium response was measured using a well established scale (Alda scale). Genotyped SNPs were used to generate data at more than 6 million sites, using standard genomic imputation methods. Traits were regressed against genotype dosage. Results were combined across two batches by meta-analysis.
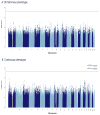
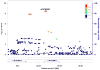
Findings: A single locus of four linked SNPs on chromosome 21 met genome-wide significance criteria for association with lithium response (rs79663003, p=1·37 × 10(-8); rs78015114, p=1·31 × 10(-8); rs74795342, p=3·31 × 10(-9); and rs75222709, p=3·50 × 10(-9)). In an independent, prospective study of 73 patients treated with lithium monotherapy for a period of up to 2 years, carriers of the response-associated alleles had a significantly lower rate of relapse than carriers of the alternate alleles (p=0·03268, hazard ratio 3·8, 95% CI 1·1-13·0).
Interpretation: The response-associated region contains two genes for long, non-coding RNAs (lncRNAs), AL157359.3 and AL157359.4. LncRNAs are increasingly appreciated as important regulators of gene expression, particularly in the CNS. Confirmed biomarkers of lithium response would constitute an important step forward in the clinical management of bipolar disorder. Further studies are needed to establish the biological context and potential clinical utility of these findings.
Funding: Deutsche Forschungsgemeinschaft, National Institute of Mental Health Intramural Research Program.
Trial registration: ClinicalTrials.gov NCT00001174.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Adli M has received a grant from Servier, speaker’s fees from Servier, Lundbecck, Aristo, Parexel, Gilead, ViiV, and Deutsche Bank plus a non-financial support from Lundbeck. Akiyama K has received speaker’s fees from Taisho Toyama Pharmaceutical. Alda M is funded by a grant of the Canadian Institutes of Health Research. Bauer M has received speaker’s fees from Alkermes, Astra Zeneca, BristolMyersSquibb, and Ferrer Internacional. Etain B received non-financial support from Labex Biopsy and Fondation Fondamental. Hashimoto R received grants and speaker honoraria from Dainippon Sumitomo Pharma and Novartis plus speaker honoraria from Eli Lilly Japan, GlaxoSmithKline, Hisamitsu Pharmaceutical, Janssen Pharmaceutical, Nippon Zoki Pharmaceutical, Otsuka Pharmaceutical, Astellas Pharma, Pfizer, and the Yoshitomiyakuhin Corporation. Kato T received a grant from Takeda Pharmaceutical and fees from Kyowa Hakko Kirin, Eli Lilly Japan, Otsuka Pharmaceutical, GlaxoSmithKline, Taisho Toyama Pharmaceutical, Dainippon Sumitomo Pharma, Meiji Seika Pharma, Pfizer Japan, Mochida Pharmaceutical, Shionogi & Co, Janssen Pharmaceutical, Yoshitomiyakuhin Corporation, Agilent Technologies, Astellas Pharma, and Wako Pure Chemical Industries. Kusumi I received grants and fees from Dainippon Sumitomo Pharma, Eisai, Eli Lilly, GlaxoSmithKline, Kyowa Hakko Kirin, Meiji Seika Pharma, MSD, Novartis, Otsuka, Ono Pharmaceutical, Pfizer, Tanabe Mitsubishi Pharma, Takeda Pharmaceutical, Shionogi, and Yoshitomi Pharmaceutical; he received grants from AbbVie GK, Asahi Kasei Pharma, Boehringer Ingelheim, Chugai Pharmaceutical, and Daiichi Sankyo and fees from Astellas Pharma and Janssen Pharmaceutical. McCarthy MJ served as unpaid consultant for Pathway Genomic (San Diego, USA). McElroy received a grant and fees from Naurex and Shire, further grants from Alkermes, Cephalon, Forest, Marriott Foundation, Orexigen Therapeutics, and Takeda Pharmaceutical, he further has served on the advisory boards for Bracket, Hoffmann-La Roche, MedAvante, Sunovion and received fees from Novo Nordisk. Perlis RH received personal fees from RID Ventures, Genomind LLC, Healthrageous, Pfizer, Perfect Health, Proteus and Psybrain. Schofield PR received a grant from NHMRC. Schulze TG received a grant and fees from Roche Pharmaceuticals. Stamm TJ received personal fees from Servier, Lundbeck and BristolMyerSquibb. All above listed interests are outside of the submitted work. All other authors declare no conflict of interests.
Figures

Comment in
-
Pharmacogenetics of lithium response: close to clinical practice?Lancet. 2016 Mar 12;387(10023):1034-1036. doi: 10.1016/S0140-6736(16)00147-1. Epub 2016 Jan 22. Lancet. 2016. PMID: 26806517 No abstract available.
References
-
- Dilsaver SC. An estimate of the minimum economic burden of bipolar I and II disorders in the United States: 2009. Journal of affective disorders. 2011;129(1–3):79–83. - PubMed
-
- Goodwin FK, Jaison KR. Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression. 2. 2007.
-
- Bauer MS, Mitchner L. What is a "mood stabilizer"? An evidence-based response The American journal of psychiatry. 2004;161(1):3–18. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- R01 MH059556/MH/NIMH NIH HHS/United States
- Z99 MH999999/ImNIH/Intramural NIH HHS/United States
- R01 MH059545/MH/NIMH NIH HHS/United States
- R01 MH059548/MH/NIMH NIH HHS/United States
- R01 MH059534/MH/NIMH NIH HHS/United States
- R01 MH59535/MH/NIMH NIH HHS/United States
- R01 MH59553/MH/NIMH NIH HHS/United States
- MR/L006642/1/MRC_/Medical Research Council/United Kingdom
- K02 DA021237/DA/NIDA NIH HHS/United States
- P50CA89392/CA/NCI NIH HHS/United States
- ZIA-MH00284311/MH/NIMH NIH HHS/United States
- R01 MH060068/MH/NIMH NIH HHS/United States
- R01 MH059535/MH/NIMH NIH HHS/United States
- R01 MH59545/MH/NIMH NIH HHS/United States
- R01 MH059567/MH/NIMH NIH HHS/United States
- R01 MH59533/MH/NIMH NIH HHS/United States
- 64410/CAPMC/ CIHR/Canada
- 5K02DA021237/DA/NIDA NIH HHS/United States
- P01 CA089392/CA/NCI NIH HHS/United States
- Z01 MH002810/ImNIH/Intramural NIH HHS/United States
- 1Z01MH002810-01/MH/NIMH NIH HHS/United States
- K02 DA21237/DA/NIDA NIH HHS/United States
- R01 MH60068/MH/NIMH NIH HHS/United States
- R01 MH059533/MH/NIMH NIH HHS/United States
- R01 MH59567/MH/NIMH NIH HHS/United States
- R01 MH059553/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

