Aqueous Angiography: Real-Time and Physiologic Aqueous Humor Outflow Imaging
- PMID: 26807586
- PMCID: PMC4725949
- DOI: 10.1371/journal.pone.0147176
Aqueous Angiography: Real-Time and Physiologic Aqueous Humor Outflow Imaging
Abstract
Purpose: Trabecular meshwork (TM) bypass surgeries attempt to enhance aqueous humor outflow (AHO) to lower intraocular pressure (IOP). While TM bypass results are promising, inconsistent success is seen. One hypothesis for this variability rests upon segmental (non-360 degrees uniform) AHO. We describe aqueous angiography as a real-time and physiologic AHO imaging technique in model eyes as a way to simulate live AHO imaging.
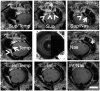
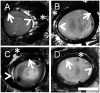
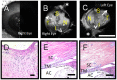
Methods: Pig (n = 46) and human (n = 6) enucleated eyes were obtained, orientated based upon inferior oblique insertion, and pre-perfused with balanced salt solution via a Lewicky AC maintainer through a 1mm side-port. Fluorescein (2.5%) was introduced intracamerally at 10 or 30 mm Hg. With an angiographer, infrared and fluorescent (486 nm) images were acquired. Image processing allowed for collection of pixel information based on intensity or location for statistical analyses. Concurrent OCT was performed, and fixable fluorescent dextrans were introduced into the eye for histological analysis of angiographically active areas.
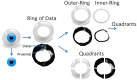
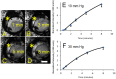
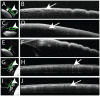
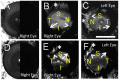
Results: Aqueous angiography yielded high quality images with segmental patterns (p<0.0001; Kruskal-Wallis test). No single quadrant was consistently identified as the primary quadrant of angiographic signal (p = 0.06-0.86; Kruskal-Wallis test). Regions of high proximal signal did not necessarily correlate with regions of high distal signal. Angiographically positive but not negative areas demonstrated intrascleral lumens on OCT images. Aqueous angiography with fluorescent dextrans led to their trapping in AHO pathways.
Conclusions: Aqueous angiography is a real-time and physiologic AHO imaging technique in model eyes.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

